Search
- Page Path
- HOME > Search
- [Korean]
- Microstructures and Mechanical Properties of Al-B4C Composites Fabricated by DED Process
- Yu-Jeong An, Ju-Yeon Han, Hyunjoo Choi, Se-Eun Shin
- J Powder Mater. 2023;30(3):262-267. Published online June 1, 2023
- DOI: https://doi.org/10.4150/KPMI.2023.30.3.262

- 1,077 View
- 9 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
PDF Boron carbide (B4C) is highly significant in the production of lightweight protective materials when added to aluminum owing to its exceptional mechanical properties. In this study, a method for fabricating Al-B4C composites using high-energy ball milling and directed energy deposition (DED) is presented. Al-4 wt.% B4C composites were fabricated under 21 different laser conditions to analyze the microstructure and mechanical properties at different values of laser power and scan speeds. The composites fabricated at a laser power of 600 W and the same scan speed exhibited the highest hardness and generated the fewest pores. In contrast, the composites fabricated at a laser power of 1000 W exhibited the lowest hardness and generated a significant number of large pores. This can be explained by the influence of the microstructure on the energy density at different values of laser power.
-
Citations
Citations to this article as recorded by- Development of Aluminum Alloys for Additive Manufacturing Using Machine Learning
Sungbin An, Juyeon Han, Seoyeon Jeon, Dowon Kim, Jae Bok Seol, Hyunjoo Choi
Journal of Powder Materials.2025; 32(3): 202. CrossRef
- Development of Aluminum Alloys for Additive Manufacturing Using Machine Learning
- [English]
- Study on Reaction Behavior of Mg-FeB Phase for Rare Earth Elements Recovery from End-of-life Magnet
- Sangmin Park, Dae-Kyeom Kim, Rongyu Liu, Jaeyun Jeong, Taek-Soo Kim, Myungsuk Song
- J Powder Mater. 2023;30(2):101-106. Published online April 1, 2023
- DOI: https://doi.org/10.4150/KPMI.2023.30.2.101
- 1,520 View
- 4 Download
-
 Abstract
Abstract
 PDF
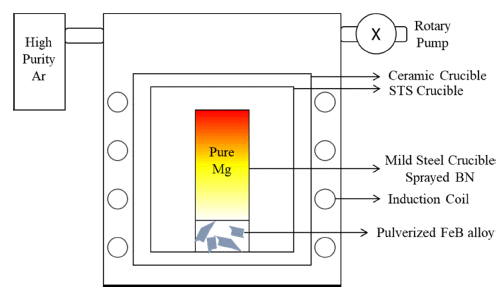
PDF Liquid metal extraction (LME), a pyrometallurgical recycling method, is popular owing to its negligible environmental impact. LME mainly targets rare-earth permanent magnets having several rare-earth elements. Mg is used as a solvent metal for LME because of its selective and eminent reactivity with rare-earth elements in magnets. Several studies concerning the formation of Dy-Fe intermetallic compounds and their effects on LME using Mg exist. However, methods for reducing these compounds are unavailable. Fe reacts more strongly with B than with Dy; B addition can be a reducing method for Dy-Fe intermetallic compounds owing to the formation of Fe2B, which takes Fe from Dy-Fe intermetallic compounds. The FeB alloy is an adequate additive for the decomposition of Fe2B. To accomplish the former process, Mg must convey B to a permanent magnet during the decomposition of the FeB alloy. Here, the effect of Mg on the transfer of B from FeB to permanent magnet is observed through microstructural and phase analyses. Through microstructural and phase analysis, it is confirmed that FeB is converted to Fe2B upon B transfer, owing to Mg. Finally, the transfer effect of Mg is confirmed, and the possibility of reducing Dy-Fe intermetallic compounds during LME is suggested.
- [Korean]
- Experimental Study on Improving Compressive Strength of Hexagonal Boron Nitride Reinforced Cement Composite
- Yomin Choi, Hyun‐Gyoo Shin
- J Korean Powder Metall Inst. 2020;27(6):503-508. Published online December 1, 2020
- DOI: https://doi.org/10.4150/KPMI.2020.27.6.503

- 651 View
- 1 Download
-
 Abstract
Abstract
 PDF
PDF The mechanical properties and microstructures of hexagonal boron nitride (h-BN)-reinforced cement composites are experimentally studied for three and seven curing days. Various sizes (5, 10, and 18 μm) and concentrations (0.1%, 0.25%, 0.5%, and 1.0%) of h-BN are dispersed by the tip ultrasonication method in water and incorporated into the cement composite. The compressive strength of the h-BN reinforced cements increases by 40.9%, when 0.5 wt% of 18 μm-sized h-BN is added. However, the compressive strength decreases when the 1.0 wt% cement composite is added, owing to the aggregation of the h-BNs in the cement composite. The microstructural characterization of the h-BN-reinforced cement composite indicates that the h-BNs act as bridges connecting the cracks, resulting in improved mechanical properties for the reinforced cement composite.
- [English]
- Synthesis of Boron Nitride Nanotubes via inductively Coupled thermal Plasma process Catalyzed by Solid-state ammonium Chloride
- Mi Se Chang, Young Gyun Nam, Sangsun Yang, Kyung Tae Kim, Ji Hun Yu, Yong-Jin Kim, Jae Won Jeong
- J Korean Powder Metall Inst. 2018;25(2):120-125. Published online April 1, 2018
- DOI: https://doi.org/10.4150/KPMI.2017.25.2.120

- 691 View
- 7 Download
-
 Abstract
Abstract
 PDF
PDF Boron nitride nanotubes (BNNTs) are receiving great attention because of their unusual material properties, such as high thermal conductivity, mechanical strength, and electrical resistance. However, high-throughput and highefficiency synthesis of BNNTs has been hindered due to the high boiling point of boron (~ 4000°C) and weak interaction between boron and nitrogen. Although, hydrogen-catalyzed plasma synthesis has shown potential for scalable synthesis of BNNTs, the direct use of H2 gas as a precursor material is not strongly recommended, as it is extremely flammable. In the present study, BNNTs have been synthesized using radio-frequency inductively coupled thermal plasma (RF-ITP) catalyzed by solid-state ammonium chloride (NH4Cl), a safe catalyst materials for BNNT synthesis. Similar to BNNTs synthesized from h-BN (hexagonal boron nitride) + H2, successful fabrication of BNNTs synthesized from h-BN+NH4Cl is confirmed by their sheet-like properties, FE-SEM images, and XRD analysis. In addition, improved dispersion properties in aqueous solution are found in BNNTs synthesized from h-BN +NH4Cl.
- [Korean]
- Synthesis of Hexagonal Boron Nitride Nanocrystals and Their Application to Thermally Conductive Composites
- Jae-Yong Jung, Yang-Do Kim, Pyung-Woo Shin, Young-Kuk Kim
- J Korean Powder Metall Inst. 2016;23(6):414-419. Published online December 1, 2016
- DOI: https://doi.org/10.4150/KPMI.2016.23.6.414

- 1,516 View
- 22 Download
-
 Abstract
Abstract
 PDF
PDF Much attention has been paid to thermally conductive materials for efficient heat dissipation of electronic devices to maintain their functionality and to support lifetime span. Hexagonal boron nitride (h-BN), which has a high thermal conductivity, is one of the most suitable materials for thermally conductive composites. In this study, we synthesize h-BN nanocrystals by pyrolysis of cost-effective precursors, boric acid, and melamine. Through pyrolysis at 900°C and subsequent annealing at 1500°C, h-BN nanoparticles with diameters of ~80 nm are synthesized. We demonstrate that the addition of small amounts of Eu-containing salts during the preparation of melamine borate precursors significantly enhanced the crystallinity of h-BN. In particular, addition of Eu assists the growth of h-BN nanoplatelets with diameters up to ~200 nm. Polymer composites containing both spherical Al2O3 (70 vol%) and Eu-doped h-BN nanoparticles (4 vol%) show an enhanced thermal conductivity (λ ~ 1.72W/mK), which is larger than the thermal conductivity of polymer composites containing spherical Al2O3 (70 vol%) as the sole fillers (λ ~ 1.48W/mK).
- [Korean]
- A Study of the Sintering Behavior of Boron Carbide using In-situ High Temperature Dilatometer
- Hyukjae Lee, Bum-Sup Kim, Tai-Joo Chung
- J Korean Powder Metall Inst. 2014;21(2):102-107. Published online April 1, 2014
- DOI: https://doi.org/10.4150/KPMI.2014.21.2.102

- 853 View
- 4 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
PDF A high temperature dilatometer attached to a graphite furnace is built and used to study the sintering behavior of B4C. Pristine and carbon doped B4C compacts are sintered at various soaking temperatures and their shrinkage profiles are detected simultaneously using the dilatometer. Carbon additions enhance the sinterability of B4C with sintering to more than 97% of the theoretical density, while pristine B4C compacts could not be sintered above 91% due to particle coarsening. The shrinkage profiles of B4C reveal that the effect of carbon on the sinterability of B4C can be seen mostly below 1950°C. The high temperature dilatometer delivers very useful information which is impossible to obtain with conventional furnaces.
-
Citations
Citations to this article as recorded by- Application of rate-controlled sintering into the study of sintering behavior of boron carbide
Hyukjae Lee
Journal of the Korean Crystal Growth and Crystal Technology.2015; 25(1): 6. CrossRef
- Application of rate-controlled sintering into the study of sintering behavior of boron carbide
TOP
 KPMI
KPMI


 First
First Prev
Prev