Search
- Page Path
- HOME > Search
- [Korean]
- Preparation of Porous W-Cu by Freeze Casting of Tert-butyl Alcohol Slurry Mixed with WO3-CuO Powder
- Youngmin Kim, Ji Young Kim, Minju Son, Wonyong Kwon, Eui Seon Lee, Sung-Tag Oh
- J Powder Mater. 2025;32(6):466-471. Published online December 31, 2025
- DOI: https://doi.org/10.4150/jpm.2025.00437
- 742 View
- 10 Download
-
 Abstract
Abstract
 PDF
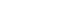
PDF - The influence of process conditions on the microstructure of porous W-Cu, fabricated by freeze casting using tert-butyl alcohol as the freezing agent, was investigated. The slurries containing 10 vol% of WO3-CuO powder were prepared by milling with a small amount of citric acid and polyethylene glycol as dispersants. The slurries with dispersion stability were frozen in a mold with the lower part cooled to -25°C, followed by sublimation in a vacuum to remove the freezing agent. The sintered W-1 vol% Cu in a hydrogen atmosphere exhibited aligned pores with the size of 50 μm, which were generated by sublimation of directionally solidified tert-butyl alcohol crystals. In the cross-section of the specimen, hexagonal pores corresponding to the crystal structure of tert-butyl alcohol was observed. Microstructure analysis of the struts revealed that Cu was distributed non-uniformly due to the mutual insolubility and low wettability of the W-Cu system.
- [Korean]
- Fabrication of Porous Tungsten by Freeze Casting and Vacuum Drying of WO3/Tert-butyl Alcohol Slurry
- Youn Ji Heo, Eui Seon Lee, Sung-Tag Oh, Young-Keun Jeong
- J Powder Mater. 2022;29(2):118-122. Published online April 1, 2022
- DOI: https://doi.org/10.4150/KPMI.2022.29.2.118

- 959 View
- 11 Download
- 3 Citations
-
 Abstract
Abstract
 PDF
PDF The synthesis of porous W by freeze-casting and vacuum drying is investigated. Ball-milled WO3 powders and tert-butyl alcohol were used as the starting materials. The tert-butyl alcohol slurry is frozen at –25°C and dried under vacuum at –25 and –10°C. The dried bodies are hydrogen-reduced at 800°C and sintered at 1000°C. The XRD analysis shows that WO3 is completely reduced to W without any reaction phases. SEM observations reveal that the struts and pores aligned in the tert-butyl alcohol growth direction, and the change in the powder content and drying temperature affects the pore structure. Furthermore, the struts of the porous body fabricated under vacuum are thinner than those fabricated under atmospheric pressure. This behavior is explained by the growth mechanism of tert-butyl alcohol and rearrangement of the powders during solidification. These results suggest that the pore structure of a porous body can be controlled by the powder content, drying temperature, and pressure.
-
Citations
Citations to this article as recorded by-
Fabrication of porous W by freeze-casting and hydrogen reduction of camphene-based WO
3
suspension
Ji Won Choi, Youngmin Kim, Ji Young Kim, Eui Seon Lee, Sung-Tag Oh
Powder Metallurgy.2025; 68(3): 283. CrossRef - Preparation of Porous W-Cu by Freeze Casting of Tert-butyl Alcohol Slurry Mixed with WO3-CuO Powder
Youngmin Kim, Ji Young Kim, Minju Son, Wonyong Kwon, Eui Seon Lee, Sung-Tag Oh
Journal of Powder Materials.2025; 32(6): 466. CrossRef - Fabrication of Porous TiO2 with Aligned Pores Using Tert-Butyl Alcohol Based Freeze Casting
Eui Seon Lee, Sung-Tag Oh
Korean Journal of Metals and Materials.2024; 62(12): 929. CrossRef
-
Fabrication of porous W by freeze-casting and hydrogen reduction of camphene-based WO
3
suspension
- [Korean]
- Effect of Freeze Drying Condition of WO3/Tert-Butyl Alcohol Slurry on the Microstructural Characteristics of Porous Body
- Eui Seon Lee, Youn Ji Heo, Myung-Jin Suk, Sung-Tag Oh
- J Korean Powder Metall Inst. 2021;28(4):331-335. Published online August 1, 2021
- DOI: https://doi.org/10.4150/KPMI.2021.28.4.331

- 621 View
- 2 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
PDF The effects of drying temperature on the microstructure of porous W fabricated by the freeze-casting process of tert-butyl alcohol slurry with WO3 powder was investigated. Green bodies were hydrogen-reduced at 800°C for 1 h and sintered at 1000°C for 6 h. X-ray diffraction analysis revealed that WO3 powders were completely converted to W without any reaction phases by hydrogen reduction. The sintered body showed pores aligned in the direction of tertbutyl alcohol growth, and the porosity and pore size decreased as the amount of WO3 increased from 5 to 10v ol%. As the drying temperature of the frozen body increased from -25°C to -10°C, the pore size and thickness of the struts increased. The change in microstructural characteristics based on the amount of powder added and the drying temperature was explained by the growth behavior of the freezing agent and the degree of rearrangement of the solid powder during the solidification of the slurry.
-
Citations
Citations to this article as recorded by- Preparation of Porous W-Cu by Freeze Casting of Tert-butyl Alcohol Slurry Mixed with WO3-CuO Powder
Youngmin Kim, Ji Young Kim, Minju Son, Wonyong Kwon, Eui Seon Lee, Sung-Tag Oh
Journal of Powder Materials.2025; 32(6): 466. CrossRef
- Preparation of Porous W-Cu by Freeze Casting of Tert-butyl Alcohol Slurry Mixed with WO3-CuO Powder
- [Korean]
- Effect of Tert-Butyl Alcohol Template on the Pore Structure of Porous Tungsten in Freeze Drying Process
- Eui Seon Lee, Youn Ji Heo, Yun Taek Ko, Jin Gyeong Park, Yong-Ho Choa, Sung-Tag Oh
- J Korean Powder Metall Inst. 2021;28(3):216-220. Published online June 1, 2021
- DOI: https://doi.org/10.4150/KPMI.2021.28.3.216

- 709 View
- 4 Download
-
 Abstract
Abstract
 PDF
PDF The effect of tert-butyl alcohol (TBA) as a freezing solvent on the pore structure of a porous tungsten body prepared by freeze-drying is analyzed. TBA slurries with a WO3 content of 10 vol% are prepared by mixing with a small amount of dispersant and binder at 30°C. The slurries are frozen at -25°C, and pores are formed in the frozen specimens by the sublimation of TBA during drying in air. After hydrogen reduction at 800°C and sintering at 1000°C, the green body of WO3 is completely converted to porous W with various pore structures. Directional pores from the center of the specimen to the outside are observed in the sintered bodies because of the columnar growth of TBA. A decrease in pore directionality and porosity is observed in the specimens prepared by long-duration drying and sintering. The change in pore structure is explained by the growth of the freezing solvent and densification.
- [Korean]
- High-Contrast Electrochromism of Porous Tungsten Oxide Thin Films Prepared by Electrodeposition
- Sung-Hyeok Park, Ho-Jin Mo, Jae-Keun Lim, Sang-Gwon Kim, Jae-Hyo Choi, Seung-Hyun Lee, Se-Hwa Jang, Kyung-Ho Cha, Yoon-Chae Nah
- J Korean Powder Metall Inst. 2018;25(1):7-11. Published online February 1, 2018
- DOI: https://doi.org/10.4150/KPMI.2018.25.1.7
- 1,059 View
- 16 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
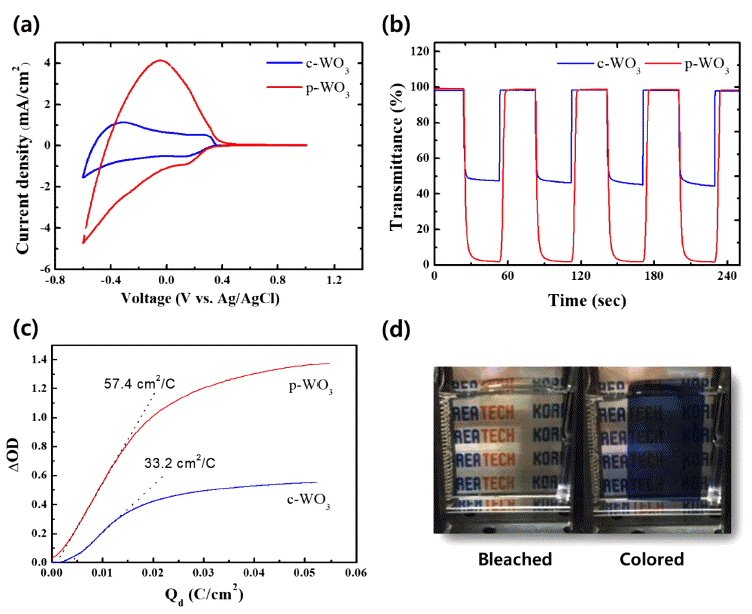
PDF In this study, we synthesize tungsten oxide thin films by electrodeposition and characterize their electrochromic properties. Depending on the deposition modes, compact and porous tungsten oxide films are fabricated on a transparent indium tin oxide (ITO) substrate. The morphology and crystal structure of the electrodeposited tungsten oxide thin films are investigated by scanning electron microscopy (SEM) and X-ray diffraction (XRD). X-ray photoelectron spectroscopy is employed to verify the chemical composition and the oxidation state of the films. Compared to the compact tungsten oxides, the porous films show superior electrochemical activities with higher reversibility during electrochemical reactions. Furthermore, they exhibit very high color contrast (97.0%) and switching speed (3.1 and 3.2 s). The outstanding electrochromic performances of the porous tungsten oxide thin films are mainly attributed to the porous structure, which facilitates ion intercalation/deintercalation during electrochemical reactions.
-
Citations
Citations to this article as recorded by- A fast-response electrochromic device based on a composite gel film comprising triphenylamine derivatives and WO3
Xuejian Zhang, Jinming Zeng, Zipeng Xu, Mimi Zhu, Ping Liu
New Journal of Chemistry.2021; 45(12): 5503. CrossRef
- A fast-response electrochromic device based on a composite gel film comprising triphenylamine derivatives and WO3
- [Korean]
- Fabrication of Porous W-Ti by Freeze-Drying and Hydrogen Reduction of WO3-TiH2 Powder Mixtures
- Hyunji Kang, Sung Hyun Park, Sung-Tag Oh
- J Korean Powder Metall Inst. 2017;24(6):472-476. Published online December 1, 2017
- DOI: https://doi.org/10.4150/KPMI.2017.24.6.472

- 623 View
- 1 Download
-
 Abstract
Abstract
 PDF
PDF Porous W-10 wt% Ti alloys are prepared by freeze-drying a WO3-TiH2/camphene slurry, using a sintering process. X-ray diffraction analysis of the heat-treated powder in an argon atmosphere shows the WO3 peak of the starting powder and reaction-phase peaks such as WO2.9, WO2, and TiO2 peaks. In contrast, a powder mixture heated in a hydrogen atmosphere is composed of the W and TiW phases. The formation of reaction phases that are dependent on the atmosphere is explained by a thermodynamic consideration of the reduction behavior of WO3 and the dehydrogenation reaction of TiH2. To fabricate a porous W-Ti alloy, the camphene slurry is frozen at -30°C, and pores are generated in the frozen specimens by the sublimation of camphene while drying in air. The green body is hydrogen-reduced and sintered at 1000°C for 1 h. The sintered sample prepared by freeze-drying the camphene slurry shows large and aligned parallel pores in the camphene growth direction, and small pores in the internal walls of the large pores. The strut between large pores consists of very fine particles with partial necking between them.
- [Korean]
- Fabrication of Porous W by Heat Treatment of Pore Forming Agent of PMMA and WO3 Powder Compacts
- Ki Cheol Jeon, Young Do Kim, Myung-Jin Suk, Sung-Tag Oh
- J Korean Powder Metall Inst. 2015;22(2):129-133. Published online April 1, 2015
- DOI: https://doi.org/10.4150/KPMI.2015.22.2.129

- 1,608 View
- 7 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
PDF Porous W with controlled pore structure was fabricated by thermal decomposition and hydrogen reduction process of PMMA beads and WO3 powder compacts. The PMMA sizes of 8 and 50 μm were used as pore forming agent for fabricating the porous W. The WO3 powder compacts with 20 and 70 vol% PMMA were prepared by uniaxial pressing and sintered for 2 h at 1200°C in hydrogen atmosphere. TGA analysis revealed that the PMMA was decomposed at about 400°C and WO3 was reduced to metallic W at 800°C. Large pores in the sintered specimens were formed by thermal decomposition of spherical PMMA, and their size was increased with increase in PMMA size and the amount of PMMA addition. Also the pore shape was changed from spherical to irregular form with increasing PMMA contents due to the agglomeration of PMMA in the powder mixing process.
-
Citations
Citations to this article as recorded by- Synthesis of Porous Silica Particles Using Sodium Silicate Precursor for Water-Repellent Surfaces
Young-Sang Cho, Nahee Ku, Young-Seok Kim
JOURNAL OF CHEMICAL ENGINEERING OF JAPAN.2019; 52(2): 194. CrossRef
- Synthesis of Porous Silica Particles Using Sodium Silicate Precursor for Water-Repellent Surfaces
TOP
 KPMI
KPMI


 First
First Prev
Prev