Search
- Page Path
- HOME > Search
- [Korean]
- A Study on the Preparation and Growth Mechanism of Titanium Dioxide using Organic-Inorganic Hybrid Titanium Complex
- Yubin Kang, Jin-Ju Choi, Nam Hun Kwon, Dae-Guen Kim, Kun-Jae Lee
- J Korean Powder Metall Inst. 2019;26(6):487-492. Published online December 1, 2019
- DOI: https://doi.org/10.4150/KPMI.2019.26.6.487
- 1,175 View
- 15 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
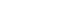
PDF Titanium dioxide (TiO2) is a typical inorganic material that has an excellent photocatalytic property and a high refractive index. It is used in water/air purifiers, solar cells, white pigments, refractory materials, semiconductors, etc.; its demand is continuously increasing. In this study, anatase and rutile phase titanium dioxide is prepared using hydroxyl and carboxyl; the titanium complex and its mechanism are investigated. As a result of analyzing the phase transition characteristics by a heat treatment temperature using a titanium complex having a hydroxyl group and a carboxyl group, it is confirmed that the material properties were different from each other and that the anatase and rutile phase contents can be controlled. The titanium complexes prepared in this study show different characteristics from the titania-formation temperatures of the known anatase and rutile phases. It is inferred that this is due to the change of electrostatic adsorption behavior due to the complexing function of the oxygen sharing point, which crystals of the TiO6 structure share.
-
Citations
Citations to this article as recorded by- Thermal Stability and Weight Reduction of Al0.75V2.82CrZr Refractory High Entropy Alloy Prepared Via Mechanical Alloying
Minsu Kim, Hansung Lee, Byungmin Ahn
journal of Korean Powder Metallurgy Institute.2023; 30(6): 478. CrossRef
- Thermal Stability and Weight Reduction of Al0.75V2.82CrZr Refractory High Entropy Alloy Prepared Via Mechanical Alloying
- [Korean]
- Recovery and Synthesis of Silver Nanoparticles from Leaching Solution of LTCC Electrode By-Products
- Juyeon Yoo, Yubin Kang, Jinju Park, Hojin Ryu, Jin-Ho Yoon, Kun-Jae Lee
- J Korean Powder Metall Inst. 2017;24(4):315-320. Published online August 1, 2017
- DOI: https://doi.org/10.4150/KPMI.2017.24.4.315

- 513 View
- 2 Download
-
 Abstract
Abstract
 PDF
PDF There has been much interest in recycling electronic wastes in order to mitigate environmental problems and to recover the large amount of constituent metals. Silver recovery from electronic waste is extensively studied because of environmental and economic benefits and the use of silver in fabricating nanodevices. Hydrometallurgical processing is often used for silver recovery because it has the advantages of low cost and ease of control. Research on synthesis recovered silver into nanoparticles is needed for application to transistors and solar cells. In this study, silver is selectively recovered from the by-product of electrodes. Silver precursors are prepared using the dissolution characteristics of the leaching solution. In the liquid reduction process, silver nanoparticles are synthesized under various surfactant conditions and then analyzed. The purity of the recovered silver is 99.24%, and the average particle size of the silver nanoparticles is 68 nm.
- [Korean]
- Dispersion Control and Characterization of the SiO2/PMMA Particles Using Surface Charge
- Yubin Kang, Soojung Son, Kun-Jae Lee
- J Korean Powder Metall Inst. 2015;22(6):403-407. Published online December 1, 2015
- DOI: https://doi.org/10.4150/KPMI.2015.22.6.403
- 1,001 View
- 7 Download
- 2 Citations
-
 Abstract
Abstract
 PDF
PDF Poly-methylmetacrylate (PMMA) is mainly applied in the plastic manufacturing industry, but PMMA is weak and gradually got discolor. The strength of PMMA can be improved through organic-inorganic hybrid nano composites with inorganic nano particles such as, SiO2 or ZrO. However, inorganic nano particles are mostly agglomerated spontaneously. In this study, the zeta potential is controlled using different types of organic solvent with different concentrations, dispersibillity of SiO2 nano particles on the PMMA particle are analyzed. When 3 M acetic acid is used, absolute value of the zeta potential is higher, SiO2 nano particle is well attached, and dispersed on the PMMA particle surface. Results indicate that the absolute value of the zeta potential affects the stability of SiO2 dispersion.
-
Citations
Citations to this article as recorded by- A Study of Organic Impurity Removal Efficiency for Waste LCD Touch Panel Glass by Solvents Types
Yubin Kang, Jin-Ju Choi, Jae Layng Park, Chan Gi Lee
Journal of the Korean Institute of Resources Recycling.2020; 29(6): 57. CrossRef - Hard Surface-adhesive Properties of TiO2 Nanoparticles-encapsulated Microparticles Prepared by Spray Drying and Surface Coating Method
Su-Kyung Kim, Jong-Duk Kim, Seung-Jun Lee
Fibers and Polymers.2018; 19(6): 1303. CrossRef
- A Study of Organic Impurity Removal Efficiency for Waste LCD Touch Panel Glass by Solvents Types
TOP
 KPMI
KPMI


 First
First Prev
Prev