Search
- Page Path
- HOME > Search
Research Article
- [Korean]
- Extraction of MgSO4 from dolomite and synthesis of Mg(OH)2 in Bittern
- HyunSeung Shim, Jiyeon Kim, Areum Choi, Nuri Oh, YooJin Kim
- J Powder Mater. 2025;32(2):122-130. Published online April 30, 2025
- DOI: https://doi.org/10.4150/jpm.2025.00073
- 952 View
- 33 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
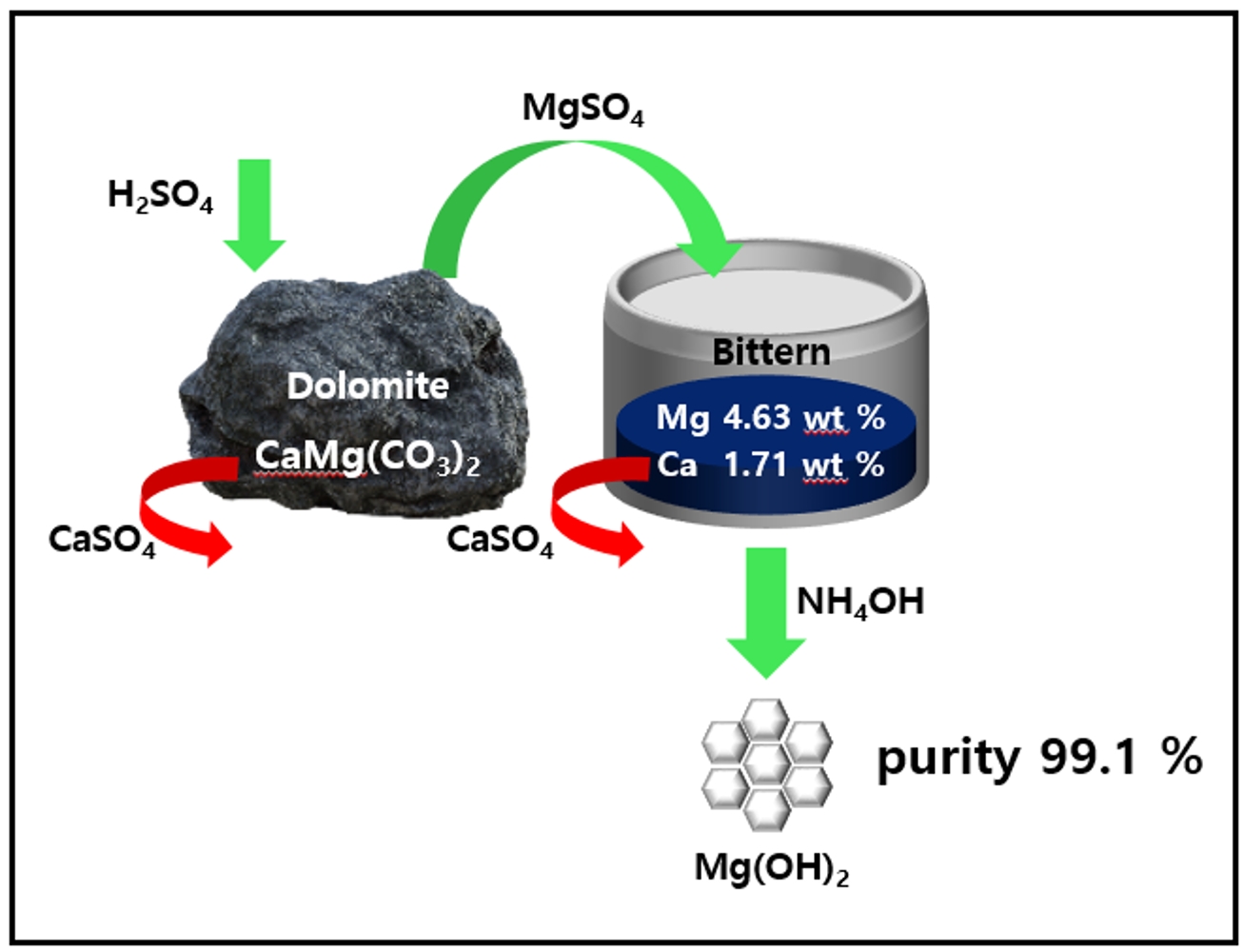
PDF - We synthesized magnesium hydroxide using bittern and dolomite, which are domestic resources. In Bittern, there is a high concentration of Mg2+ ions, but the impurity Ca2+ ion content is also significant, requiring a purification process to remove it. There are two main methods for this purification. Firstly, there is a separation method that utilizes the difference in solubility between Mg2+ ions and Ca2+ ions by using sulfuric acid on dolomite. Adding MgSO4 solution from dolomite to Bittern removes Ca2+ ions as CaSO4. This process simultaneously purifies Ca impurities and increases the Mg/Ca ratio by adding extra Mg2+ ions. In this study, purified bittern was obtained by using dolomite and sulfuric acid to extract MgSO4, which was then used to purify Ca2+ ions. High-purity Mg(OH)2 was synthesized by optimizing the NaOH and NH4OH ratio as an alkaline precipitant.
-
Citations
Citations to this article as recorded by- Synthesis and Morphology Control of Needle Type 513 MHSH and Mg(OH)2 from Dolomite
Jiyeon Kim, HyunSeung Shim, Seong-Ju Hwang, YooJin Kim
Journal of Powder Materials.2025; 32(5): 399. CrossRef
- Synthesis and Morphology Control of Needle Type 513 MHSH and Mg(OH)2 from Dolomite
TOP
 KPMI
KPMI


 First
First Prev
Prev