Search
- Page Path
- HOME > Search
- [English]
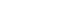
- Thermodynamic and Electronic Descriptor-Driven Machine Learning for Phase Prediction in High-Entropy Alloys: Experimental Validation
- Nguyen Lam Khoa, Nguyen Duy Khanh, Hoang Thi Ngoc Quyen, Nguyen Thi Hoang Oanh, , Le Hong Thang, Nguyen Hoa Khiem, Nguyen Hoang Viet
- J Powder Mater. 2025;32(3):191-201. Published online June 30, 2025
- DOI: https://doi.org/10.4150/jpm.2025.00143
- 2,469 View
- 82 Download
- 3 Citations
-
 Abstract
Abstract
 PDF
PDF - High-entropy alloys (HEAs) exhibit complex phase formation behavior, challenging conventional predictive methods. This study presents a machine learning (ML) framework for phase prediction in HEAs, using a curated dataset of 648 experimentally characterized compositions and features derived from thermodynamic and electronic descriptors. Three classifiers—random forest, gradient boosting, and CatBoost—were trained and validated through cross-validation and testing. Gradient boosting achieved the highest accuracy, and valence electron concentration (VEC), atomic size mismatch (δ), and enthalpy of mixing (ΔHmix) were identified as the most influential features. The model predictions were experimentally verified using a non-equiatomic Al₃₀Cu₁₇.₅Fe₁₇.₅Cr₁₇.₅Mn₁₇.₅ alloy and the equiatomic Cantor alloy (CoCrFeMnNi), both of which showed strong agreement with predicted phase structures. The results demonstrate that combining physically informed feature engineering with ML enables accurate and generalizable phase prediction, supporting accelerated HEA design.
-
Citations
Citations to this article as recorded by- Effect of annealing temperature on thermal expansion and cryogenic mechanical properties of low-thermal-expansion Co22.2Cr6.2Fe48.8Ni17.8Cu5.0 medium-entropy alloy
Wooyoung Lee, Munsu Choi, Sungwook Kim, Dae-Kyeom Kim, Myungsuk Song, Taek-Soo Kim, Jungwan Lee, Hyoung Seop Kim, Hyunjoo Choi, Soo-Hyun Joo
Materials Science and Engineering: A.2026; 954: 149811. CrossRef - Preparation and Arc Erosion Behavior of Cu-Based Contact Materials Reinforced with High Entropy Particles CuCrNiCoFe
Jiacheng Tong, Jun Wang, Huimin Zhang, Haoran Liu, Youchang Sun, Zhiguo Li, Wenyi Zhang, Zhe Wang, Yanli Chang, Zhao Yuan, Henry Hu
Metallurgical and Materials Transactions B.2025; 56(5): 5948. CrossRef - Recent progresses on high entropy alloy development using machine learning: A review
Abhishek Kumar, Nilay Krishna Mukhopadhyay, Thakur Prasad Yadav
Computational Materials Today.2025; 8: 100038. CrossRef
- Effect of annealing temperature on thermal expansion and cryogenic mechanical properties of low-thermal-expansion Co22.2Cr6.2Fe48.8Ni17.8Cu5.0 medium-entropy alloy
- [English]
- Effect of Calcium Addition on the High-Temperature Recovery of Nd and Dy from Nd-Fe-B Scrap Using Mg-Based Extractants
- Hyoseop Kim
- J Powder Mater. 2024;31(6):493-499. Published online December 31, 2024
- DOI: https://doi.org/10.4150/jpm.2024.00283

- 1,716 View
- 18 Download
-
 Abstract
Abstract
 PDF
PDF - This study investigated whether calcium (Ca) addition improved the recovery of neodymium (Nd) and dysprosium (Dy) from Nd-Fe-B magnet scrap using magnesium (Mg)-based liquid metal extraction (LME). Traditional LME processes are limited to temperatures up to 850 °C due to oxidation issues, reducing the efficiency of rare earth element (REE) recovery, especially for Dy. By adding 10 wt.% Ca to Mg and increasing the processing temperature to 1,000 °C, we achieved nearly 100% Nd and approximately 38% Dy recovery, compared to 91% and 28%, respectively, with pure Mg at 850 °C. However, excessive Ca addition (20 wt.%) decreased the recovery efficiency due to the formation of stable intermetallic compounds. These results highlight the critical role of Ca in optimizing REE recycling from Nd-Fe-B magnet scrap.
- [English]
- Study on Reaction Behavior of Mg-FeB Phase for Rare Earth Elements Recovery from End-of-life Magnet
- Sangmin Park, Dae-Kyeom Kim, Rongyu Liu, Jaeyun Jeong, Taek-Soo Kim, Myungsuk Song
- J Powder Mater. 2023;30(2):101-106. Published online April 1, 2023
- DOI: https://doi.org/10.4150/KPMI.2023.30.2.101
- 1,523 View
- 4 Download
-
 Abstract
Abstract
 PDF
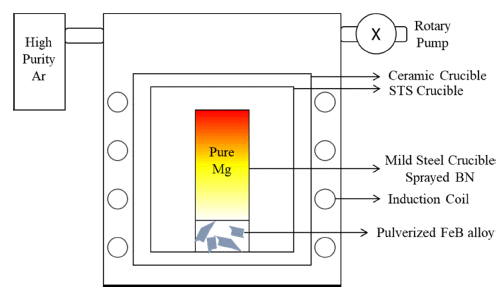
PDF Liquid metal extraction (LME), a pyrometallurgical recycling method, is popular owing to its negligible environmental impact. LME mainly targets rare-earth permanent magnets having several rare-earth elements. Mg is used as a solvent metal for LME because of its selective and eminent reactivity with rare-earth elements in magnets. Several studies concerning the formation of Dy-Fe intermetallic compounds and their effects on LME using Mg exist. However, methods for reducing these compounds are unavailable. Fe reacts more strongly with B than with Dy; B addition can be a reducing method for Dy-Fe intermetallic compounds owing to the formation of Fe2B, which takes Fe from Dy-Fe intermetallic compounds. The FeB alloy is an adequate additive for the decomposition of Fe2B. To accomplish the former process, Mg must convey B to a permanent magnet during the decomposition of the FeB alloy. Here, the effect of Mg on the transfer of B from FeB to permanent magnet is observed through microstructural and phase analyses. Through microstructural and phase analysis, it is confirmed that FeB is converted to Fe2B upon B transfer, owing to Mg. Finally, the transfer effect of Mg is confirmed, and the possibility of reducing Dy-Fe intermetallic compounds during LME is suggested.
- [Korean]
- Oxidation Behaviors and Degradation Properties of Aluminide Coated Stainless Steel at High Temperature
- Cheol Hong Hwang, Hyo Min Lee, Jeong Seok Oh, Dong Hyeon Hwang, Yu Seok Hwang, Jong Won Lee, Jeong Mook Choi, Joon Sik Park
- J Korean Powder Metall Inst. 2021;28(5):396-402. Published online October 1, 2021
- DOI: https://doi.org/10.4150/KPMI.2021.28.5.396

- 765 View
- 2 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
PDF Stainless steel, a type of steel used for high-temperature parts, may cause damage when exposed to high temperatures, requiring additional coatings. In particular, the Cr2O3 product layer is unstable at 1000°C and higher temperatures; therefore, it is necessary to improve the oxidation resistance. In this study, an aluminide (Fe2Al5 and FeAl3) coating layer was formed on the surface of STS 630 specimens through Al diffusion coatings from 500°C to 700°C for up to 25 h. Because the coating layers of Fe2Al5 and FeAl3 could not withstand temperatures above 1200°C, an Al2O3 coating layer is deposited on the surface through static oxidation treatment at 500°C for 10 h. To confirm the ablation resistance of the resulting coating layer, dynamic flame exposure tests were conducted at 1350°C for 5–15 min. Excellent oxidation resistance is observed in the coated base material beneath the aluminide layer. The conditions of the flame tests and coating are discussed in terms of microstructural variations.
-
Citations
Citations to this article as recorded by- Thermal Stability and Degradation Properties of Aluminide Coated and Uncoated Ti-6Al-4V Alloys Exposed to High Temperature Flame
C. Hwang, J. Park, J. Oh, D. Han, S. Lee, K. Shin, J. Choi, K. P. Shinde, J. S. Park
Metals and Materials International.2023; 29(7): 1855. CrossRef
- Thermal Stability and Degradation Properties of Aluminide Coated and Uncoated Ti-6Al-4V Alloys Exposed to High Temperature Flame
- [English]
- Effect of Oxidation Behavior of (Nd,Dy)-Fe-B Magnet on Heavy Rare Earth Extraction Process
- Sangmin Park, Sun-Woo Nam, Sang-Hoon Lee, Myung-Suk Song, Taek-Soo Kim
- J Korean Powder Metall Inst. 2021;28(2):91-96. Published online April 1, 2021
- DOI: https://doi.org/10.4150/KPMI.2021.28.2.91
- 1,812 View
- 21 Download
- 6 Citations
-
 Abstract
Abstract
 PDF
PDF Rare earth magnets with excellent magnetic properties are indispensable in the electric device, wind turbine, and e-mobility industries. The demand for the development of eco-friendly recycling techniques has increased to realize sustainable green technology, and the supply of rare earth resources, which are critical for the production of permanent magnets, are limited. Liquid metal extraction (LME), which is a type of pyrometallurgical recycling, is known to selectively extract the metal forms of rare earth elements. Although several studies have been carried out on the formation of intermetallic compounds and oxides, the effect of oxide formation on the extraction efficiency in the LME process remains unknown. In this study, microstructural and phase analyses are conducted to confirm the oxidation behavior of magnets pulverized by a jaw crusher. The LME process is performed with pulverized scrap, and extraction percentages are calculated to confirm the effect of the oxide phases on the extraction of Dy during the reaction. During the LME p rocess, Nd i s completely e xtracted a fter 6 h, w hile D y remains as D y2Fe17 and Dy-oxide. Because the decomposition rate of Dy2Fe17 is faster than the reduction rate of Dy-oxide, the importance of controlling Dy-oxide on Dy extraction is confirmed.
-
Citations
Citations to this article as recorded by- Manipulation of reactivity based on metallic adsorption in magnesium alloy scraps for rare-earth recycling by liquid metal extraction
Sangmin Park, Yoonhyung Keum, Jaeyun Jeong, Seunghun Cha, Ju-Young Cho, Hyunchul Kim, Jiseong Lee, Taek-Soo Kim, Dae-Kyeom Kim, Myungsuk Song
Journal of Alloys and Compounds.2025; 1022: 178711. CrossRef - A Review of the Current Progress in High-Temperature Recycling Strategies for Recovery of Rare-Earth Elements from Magnet Waste
Ali Zakeri, Leili Tafaghodi
Journal of Sustainable Metallurgy.2025; 11(1): 88. CrossRef - Selective growth of Nb–Fe–B intermetallic compounds for the direct separation of rare earths based on manipulating liquation
Sangmin Park, Jaeyun Jeong, Seunghun Cha, Yoonhyung Keum, Ju-Young Cho, Hyungbeen Park, Taek-Soo Kim, Dae-Kyeom Kim, Myungsuk Song
Materials Today Sustainability.2024; 28: 101042. CrossRef - Separation and recovery Nd and Dy from Mg-REEs alloy by vacuum distillation
Sangmin Park, Dae-Kyeom Kim, Jaeyun Jeong, Jae Hong Shin, Yujin Kang, Rongyu Liu, Taek-Soo Kim, Myungsuk Song
Journal of Alloys and Compounds.2023; 967: 171775. CrossRef - The Supported Boro-Additive Effect for the Selective Recovery of Dy Elements from Rare-Earth-Elements-Based Magnets
Sangmin Park, Dae-Kyeom Kim, Javid Hussain, Myungsuk Song, Taek-Soo Kim
Materials.2022; 15(9): 3032. CrossRef - Influence of Dysprosium Compounds on the Extraction Behavior of Dy from Nd-Dy-Fe-B Magnet Using Liquid Magnesium
Sun-Woo Nam, Sang-Min Park, Mohammad Zarar Rasheed, Myung-Suk Song, Do-Hyang Kim, Taek-Soo Kim
Metals.2021; 11(9): 1345. CrossRef
- Manipulation of reactivity based on metallic adsorption in magnesium alloy scraps for rare-earth recycling by liquid metal extraction
- [Korean]
- Research Trends of High-entropy Alloys
- Pureunsol Park, Ho Joon Lee, Youngjun Jo, Bonseung Gu, Won June Choi, Jongmin Byun
- J Korean Powder Metall Inst. 2019;26(6):515-527. Published online December 1, 2019
- DOI: https://doi.org/10.4150/KPMI.2019.26.6.515
- 5,717 View
- 44 Download
- 2 Citations
-
 Abstract
Abstract
 PDF
PDF High-entropy alloys (HEAs) are generally defined as solid solutions containing at least 5 constituent elements with concentrations between 5 and 35 atomic percent without the formation of intermetallic compounds. Currently, HEAs receive great attention as promising candidate materials for extreme environments due to their potentially desirable properties that result from their unique structural properties. In this review paper, we aim to introduce HEAs and explain their properties and related research by classifying them into three main categories, namely, mechanical properties, thermal properties, and electrochemical properties. Due to the high demand for structural materials in extreme environments, the mechanical properties of HEAs including strength, hardness, ductility, fatigue, and wear resistance are mainly described. Thermal and electrochemical properties, essential for the application of these alloys as structural materials, are also described.
-
Citations
Citations to this article as recorded by- Composites of equiatomic Y, La, Ce, Nd, and Gd rare earth oxides: Chemical-shift effects and valence spectra
Jungsu Bin, Hyunbae Gee, Taesung Park, UiJun Go, Jeoung Han Kim, Youn-Seoung Lee
Current Applied Physics.2024; 59: 85. CrossRef - Sintering Behavior and Mechanical Property of Transition Metal Carbide-Based Cermets by Spark Plasma Sintering
Jeong-Han Lee, Hyun-Kuk Park, Sung-Kil Hong
Korean Journal of Materials Research.2022; 32(1): 44. CrossRef
- Composites of equiatomic Y, La, Ce, Nd, and Gd rare earth oxides: Chemical-shift effects and valence spectra
- [Korean]
- Synthesis of Nanopowders by Hydrothermal Method and their Application to Dye-sentisized Solar Cell Materials
- JinYoung Lim, Jeongseok Ahn, Jung-Ho Ahn
- J Korean Powder Metall Inst. 2018;25(4):309-315. Published online August 1, 2018
- DOI: https://doi.org/10.4150/KPMI.2018.25.4.309

- 694 View
- 2 Download
-
 Abstract
Abstract
 PDF
PDF In the present work, we synthesize nano-sized ZnO, SnO2, and TiO2 powders by hydrothermal reaction using metal chlorides. We also examine the energy-storage characteristics of the resulting materials to evaluate the potential application of these powders to dye-sensitized solar cells. The control of processing parameters such as pressure, temperature, and the concentration of aqueous solution results in the formation of a variety of powder morphologies with different sizes. Nano-rod, nano-flower, and spherical powders are easily formed with the present method. Heat treatment after the hydrothermal reaction usually increases the size of the powder. At temperatures above 1000°C, a complete collapse of the shape occurs. With regard to the capacity of DSSC materials, the hydrothermally synthesized TiO2 results in the highest current density of 9.1 mA/cm2 among the examined oxides. This is attributed to the fine particle size and morphology with large specific surface area.
- [Korean]
- Fabrication of Uniform TiO2 Blocking Layers for Prevention of Electron Recombination in Dye-Sensitized Solar Cells
- Ju-won Bae, Bon-Ryul Koo, Tae-Kuen Lee, Hyo-Jin Ahn
- J Korean Powder Metall Inst. 2018;25(1):1-6. Published online February 1, 2018
- DOI: https://doi.org/10.4150/KPMI.2018.25.1.1
- 765 View
- 1 Download
- 3 Citations
-
 Abstract
Abstract
 PDF
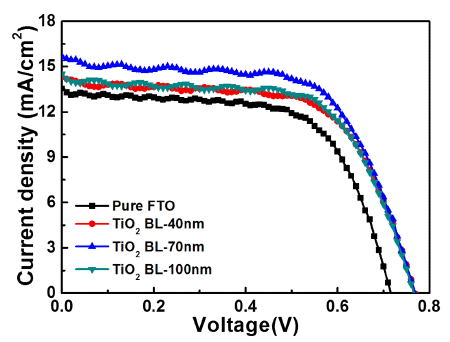
PDF Uniform TiO2 blocking layers (BLs) are fabricated using ultrasonic spray pyrolysis deposition (USPD) method. To improve the photovoltaic performance of dye-sensitized solar cells (DSSCs), the BL thickness is controlled by using USPD times of 0, 20, 60, and 100 min, creating TiO2 BLs of 0, 40, 70, and 100 nm, respectively, in average thickness on fluorine-doped tin oxide (FTO) glass. Compared to the other samples, the DSSC containing the uniform TiO2 BL of 70 nm in thickness shows a superior power conversion efficiency of 7.58±0.20% because of the suppression of electron recombination by the effect of the optimized thickness. The performance improvement is mainly attributed to the increased open-circuit voltage (0.77±0.02 V) achieved by the increased Fermi energy levels of the working electrodes and the improved short-circuit current density (15.67±0.43 mA/cm2) by efficient electron transfer pathways. Therefore, optimized TiO2 BLs fabricated by USPD may allow performance improvements in DSSCs.
-
Citations
Citations to this article as recorded by- Flexible Dye-sensitized Solar Cell Using Titanium Gel at Low Temperature
Seung Hwan Ji, Hyunsu Park, Doyeon Kim, Do Hyung Han, Hye Won Yun, Woo-Byoung Kim
Korean Journal of Materials Research.2019; 29(3): 183. CrossRef - Surface tailoring of zinc electrodes for energy storage devices with high-energy densities and long cycle life
Geon-Hyoung An, SeungNam Cha, Jung Inn Sohn
Applied Surface Science.2019; 467-468: 1157. CrossRef - Crystallinity Control Effects on Vanadium Oxide Films for Enhanced Electrochromic Performances
Kue-Ho Kim, Ju-Won Bae, Tae-Kuen Lee, Hyo-Jin Ahn
Korean Journal of Materials Research.2019; 29(6): 385. CrossRef
- Flexible Dye-sensitized Solar Cell Using Titanium Gel at Low Temperature
- [Korean]
- Photocatalytic and Adsorption Properties of WO3 Nanorods Prepared by Hydrothermal Synthesis
- Su-Yeol Yu, Chunghee Nam
- J Korean Powder Metall Inst. 2017;24(6):483-488. Published online December 1, 2017
- DOI: https://doi.org/10.4150/KPMI.2017.24.6.483

- 1,173 View
- 4 Download
- 2 Citations
-
 Abstract
Abstract
 PDF
PDF Transition-metal oxide semiconductors have various band gaps. Therefore, many studies have been conducted in various application fields. Among these, methods for the adsorption of organic dyes and utilization of photocatalytic properties have been developed using various metal oxides. In this study, the adsorption and photocatalytic effects of WO3 nanomaterials prepared by hydrothermal synthesis are investigated, with citric acid added in the hydrothermal process as a structure-directing agent. The nanostructures of WO3 are studied using transmission electron microscopy and scanning electron microscopy images. The crystal structure is investigated using X-ray diffraction patterns, and the changes in the dye concentrations adsorbed on WO3 nanorods are measured with a UV-visible absorption spectrophotometer based on Beer-Lambert’s law. The methylene blue (MB) dye solution is subjected to acid or base conditions to monitor the change in the maximum adsorption amount in relation to the pH. The maximum adsorption capacity is observed at pH 3. In addition to the dye adsorption, UV irradiation is carried out to investigate the decomposition of the MB dye as a result of photocatalytic effects. Significant photocatalytic properties are observed and compared with the adsorption effects for dye removal.
-
Citations
Citations to this article as recorded by- Photocatalytic Properties of WO3 Thin Films Prepared by Electrodeposition Method
Kwang-Mo Kang, Ji-Hye Jeong, Ga-In Lee, Jae-Min Im, Hyun-Jeong Cheon, Deok-Hyeon Kim, Yoon-Chae Nah
Journal of Korean Powder Metallurgy Institute.2019; 26(1): 40. CrossRef - Photocatalysis of TiO<sub>2</sub>/WO<sub>3</sub> Composites Synthesized by Ball Milling
Su-Yeol Yu, Chunghee Nam
Journal of Korean Powder Metallurgy Institute.2018; 25(4): 316. CrossRef
- Photocatalytic Properties of WO3 Thin Films Prepared by Electrodeposition Method
- [English]
- Magnetically Driven Assemblies of γ-Fe3O4 Nanoparticles into Well-Ordered Permanent Structures
- Myunghwan Byun
- J Korean Powder Metall Inst. 2017;24(3):229-234. Published online June 1, 2017
- DOI: https://doi.org/10.4150/KPMI.2017.24.3.229

- 862 View
- 1 Download
-
 Abstract
Abstract
 PDF
PDF We report on a simple and robust route to the spontaneous assembly of well-ordered magnetic nanoparticle superstructures by irreversible evaporation of a sessile single droplet of a mixture of a ferrofluid (FF) and a nonmagnetic fluid (NF). The resulting assembled superstructures are seen to form well-packed, vertically arranged columns with diameters of 5~0.7 μm, interparticle spacings of 9~2 μm, and heights of 1.3~3 μm. The assembled superstructures are strongly dependent on both the magnitude of magnetic field and the mixing ratio of the mixture. As the magnitude of the externally applied magnetic field and the mixing ratio of the mixture increase gradually, the size and interspacing of the magnetic nanoparticle aggregations decrease. Without an externally applied magnetic field, featureless patterns are observed for the γ-Fe3O4 nanoparticle aggregations. The proposed approach may lead to a versatile, cost-effective, fast, and scalable fabrication process based on the field-induced self-assembly of magnetic nanoparticles.
- [Korean]
- Synthesis of DyF3 paste and Magnetic Properties of GBDPed Nd-Fe-B Magnets
- Kwang-Won Jeon, Hee-Ryoung Cha, Jung-Goo Lee
- J Korean Powder Metall Inst. 2016;23(6):437-441. Published online December 1, 2016
- DOI: https://doi.org/10.4150/KPMI.2016.23.6.437

- 624 View
- 4 Download
-
 Abstract
Abstract
 PDF
PDF Recently, the grain boundary diffusion process (GBDP), involving heavy rare-earth elements such as Dy and Tb, has been widely used to enhance the coercivity of Nd-Fe-B permanent magnets. For example, a Dy compound is coated onto the surface of Nd-Fe-B sintered magnets, and then the magnets are heat treated. Subsequently, Dy diffuses into the grain boundaries of Nd-Fe-B magnets, forming Dy-Fe-B or Nd-Dy-Fe-B. The dip-coating process is also used widely instead of the GBDP. However, it is quite hard to control the thickness uniformity using dip coating. In this study, first, a DyF3 paste is fabricated using DyF3 powder. Subsequently, the fabricated DyF3 paste is homogeneously coated onto the surface of a Nd-Fe-B sintered magnet. The magnet is then subjected to GBDP to enhance its coercivity. The weight ratio of binder and DyF3 powder is controlled, and we find that the coercivity enhances with decreasing binder content. In addition, the maximum coercivity is obtained with the paste containing 70 wt% of DyF3 powder.
- [Korean]
- Effect of Cu/Al powder mixing on Dy diffusion in Nd-Fe-B sintered magnets treated with a grain boundary diffusion process
- Min Woo Lee, Tae Suk Jang
- J Korean Powder Metall Inst. 2016;23(6):432-436. Published online December 1, 2016
- DOI: https://doi.org/10.4150/KPMI.2016.23.6.432

- 802 View
- 6 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
PDF We investigate the microstructural and magnetic property changes of DyH2, Cu + DyH2, and Al + DyH2 diffusion-treated NdFeB sintered magnets with the post annealing (PA) temperature. The coercivity of all the diffusiontreated magnets increases with increasing heat treatment temperature except at 910°C, where it decreases slightly. Moreover, at 880°C, the coercivity increases by 3.8 kOe in Cu and 4.7 kOe in Al-mixed DyH2-coated magnets, whereas this increase is relatively low (3.0 kOe) in the magnet coated with only DyH2. Both Cu and Al have an almost similar effect on the coercivity improvement, particularly over the heat treatment temperature range of 790-880°C. The diffusivity and diffusion depth of Dy increases in those magnets that are treated with Cu or Al-mixed DyH2, mainly because of the comparatively easy diffusion path provided by Cu and Al owing to their solubility in the Nd-rich grain boundary phase. The formation of a highly anisotropic (Nd, Dy)2Fe14B phase layer, which acts as the shell in the core-shell-type structure so as to prevent the reverse domain movement, is the cause of enhanced coercivity of diffusion-treated Nd-Fe-B magnets.
-
Citations
Citations to this article as recorded by- Synthesize of Nd2Fe14B Powders from 1-D Nd2Fe14B Wires using Electrospinning Process
Nu Si A Eom, Su Noh, Muhammad Aneeq Haq, Bum Sung Kim
Journal of Korean Powder Metallurgy Institute.2019; 26(6): 477. CrossRef
- Synthesize of Nd2Fe14B Powders from 1-D Nd2Fe14B Wires using Electrospinning Process
- [Korean]
- Fabrication of Ti Porous body with Improved Specific Surface Area by Synthesis of CNTs
- Hye Rim Choi, Jong Min Byun, Myung-Jin Suk, Sung-Tag Oh, Young Do Kim
- J Korean Powder Metall Inst. 2016;23(3):235-239. Published online June 1, 2016
- DOI: https://doi.org/10.4150/KPMI.2016.23.3.235

- 494 View
- 0 Download
-
 Abstract
Abstract
 PDF
PDF This study is performed to fabricate a Ti porous body by freeze drying process using titanium hydride (TiH2) powder and camphene. Then, the Ti porous body is employed to synthesize carbon nanotubes (CNTs) using thermal catalytic chemical vapor deposition (CCVD) with Fe catalyst and methane (CH4) gas to increase the specific surface area. The synthesized Ti porous body has 100 μm-sized macropores and 10-30 μm-sized micropores. The synthesized CNTs have random directions and are entangled with adjacent CNTs. The CNTs have a bamboo-like structure, and their average diameter is about 50 nm. The Fe nano-particles observed at the tip of the CNTs indicate that the tip growth model is applicable. The specific surface area of the CNT-coated Ti porous body is about 20 times larger than that of the raw Ti porous body. These CNT-coated Ti porous bodies are expected to be used as filters or catalyst supports.
- [Korean]
- Spindle-shaped Fe2O3 Nanoparticle Coated Carbon Nanofiber Composites for Low-cost Dye-sensitized Solar Cells
- Dong-Hyeun Oh, HyeLan An, Bon-Ryul Koo, Hyo-Jin Ahn
- J Korean Powder Metall Inst. 2016;23(2):95-101. Published online April 1, 2016
- DOI: https://doi.org/10.4150/KPMI.2016.23.2.95

- 1,055 View
- 3 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
PDF Carbon nanofiber (CNF) composites coated with spindle-shaped Fe2O3 nanoparticles (NPs) are fabricated by a combination of an electrospinning method and a hydrothermal method, and their morphological, structural, and chemical properties are measured by field-emission scanning electron microscopy, transmission electron microscopy, Xray diffraction, and X-ray photoelectron spectroscopy. For comparison, CNFs and spindle-shaped Fe2O3 NPs are prepared by either an electrospinning method or a hydrothermal method, respectively. Dye-sensitized solar cells (DSSCs) fabricated with the composites exhibit enhanced open circuit voltage (0.70 V), short-circuit current density (12.82 mA/cm2), fill factor (61.30%), and power conversion efficiency (5.52%) compared to those of the CNFs (0.66 V, 11.61 mA/cm2, 51.96%, and 3.97%) and spindle-shaped Fe2O3 NPs (0.67 V, 11.45 mA/cm2, 50.17%, and 3.86%). This performance improvement can be attributed to a synergistic effect of a superb catalytic reaction of spindle-shaped Fe2O3 NPs and efficient charge transfer relative to the one-dimensional nanostructure of the CNFs. Therefore, spindle-shaped Fe2O3-NPcoated CNF composites may be proposed as a potential alternative material for low-cost counter electrodes in DSSCs.
-
Citations
Citations to this article as recorded by- Ni Nanoparticles-Graphitic Carbon Nanofiber Composites for Pt-Free Counter Electrode in Dye-Sensitized Solar Cells
Dong-Hyeun Oh, Bon-Ryul Koo, Yu-Jin Lee, HyeLan An, Hyo-Jin Ahn
Korean Journal of Materials Research.2016; 26(11): 649. CrossRef
- Ni Nanoparticles-Graphitic Carbon Nanofiber Composites for Pt-Free Counter Electrode in Dye-Sensitized Solar Cells
- [Korean]
- The Preparation of Dye-Sensitized Solar Cell Paste Used the Peroxo Titanium Complex and Characteristics by Annealing Temperature
- Hyunsu Park, Soyeong Joo, Joon-Phil Choi, Woo-Byoung Kim
- J Korean Powder Metall Inst. 2015;22(6):396-402. Published online December 1, 2015
- DOI: https://doi.org/10.4150/KPMI.2015.22.6.396

- 1,020 View
- 6 Download
- 5 Citations
-
 Abstract
Abstract
 PDF
PDF The organic binder-free paste for dye-sensitized solar cell (DSSC) has been investigated using peroxo titanium complex. The crystal structure of TiO2 nanoparticles, morphology of TiO2 film and electrical properties are analyzed by X-Ray Diffraction (XRD), Scanning Electron Microscopy (SEM), Electrochemical Impedance Spectra (EIS), and solar simulator. The synthesized TiO2 nanopowders by the peroxo titanium complex at 150, 300, 400°C, and 450°C have anatase phase and average crystal sizes are calculated to be 4.2, 13.7, 16.9, and 20.9 nm, respectively. The DSSC prepared by the peroxo titanium complex binder have higher Voc and lower Jsc values than that of the organic binder. It can be attributed to improvement of sintering properties of TCO/TiO2 and TiO2/TiO2 interface and to formation of agglomerate by the nanoparticles. As a result, we have investigated the organic binder-free paste and 3.178% conversion efficiency of the DSSC at 450°C.
-
Citations
Citations to this article as recorded by- Development of Eco-Friendly Ag Embedded Peroxo Titanium Complex Solution Based Thin Film and Electrical Behaviors of Resistive Random Access Memory
Won Jin Kim, Jinho Lee, Ryun Na Kim, Donghee Lee, Woo-Byoung Kim
Korean Journal of Materials Research.2024; 34(3): 152. CrossRef - Development of eco-friendly thin film manufacturing process using poeroxo titanium complex solution and potential for resistive random access memory
Jinho Lee, Ryun Na Kim, Kee-Ryung Park, Woo-Byoung Kim
Applied Surface Science.2021; 562: 150170. CrossRef - Preparation of ultra-thin TiO2 shell by peroxo titanium complex (PTC) solution-based green surface modification, and photocatalytic activity of homo-core/shell TiO2
Jinho Lee, Jiyong Hwang, Hyunsu Park, Tohru Sekino, Woo-Byoung Kim
Applied Surface Science.2021; 540: 148399. CrossRef - Effects of Annealing Temperature on the Crystal Structure, Morphology, and Optical Properties of Peroxo-Titanate Nanotubes Prepared by Peroxo-Titanium Complex Ion
Hyunsu Park, Tomoyo Goto, Sunghun Cho, Soo Wohn Lee, Masato Kakihana, Tohru Sekino
Nanomaterials.2020; 10(7): 1331. CrossRef - Study on thermal behavior of Ammonium Hexafluofide Titanate for Synthesis of TiO2 Powders
Duk-Hee Lee, Jae-Ryang Park, Chan-Gi Lee, Kyung-Soo Park, Hyeon-Mo Kim
Journal of Korean Powder Metallurgy Institute.2016; 23(5): 353. CrossRef
- Development of Eco-Friendly Ag Embedded Peroxo Titanium Complex Solution Based Thin Film and Electrical Behaviors of Resistive Random Access Memory
- [English]
- Atomistic Simulation of Sintering Mechanism for Copper Nano-Powders
- Yujin Seong, Sungwon Hwang, See Jo Kim, Sungho Kim, Seong-Gon Kim, Hak Jun Kim, Seong Jin Park
- J Korean Powder Metall Inst. 2015;22(4):247-253. Published online August 1, 2015
- DOI: https://doi.org/10.4150/KPMI.2015.22.4.247
- 1,605 View
- 12 Download
- 3 Citations
-
 Abstract
Abstract
 PDF
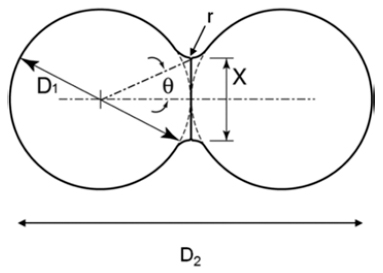
PDF The sintering mechanisms of nanoscale copper powders have been investigated. A molecular dynamics (MD) simulation with the embedded-atom method (EAM) was employed for these simulations. The dimensional changes for initial-stage sintering such as characteristic lengths, neck growth, and neck angle were calculated to understand the densification behavior of copper nano-powders. Factors affecting sintering such as the temperature, powder size, and crystalline misalignment between adjacent powders have also been studied. These results could provide information of setting the processing cycles and material designs applicable to nano-powders. In addition, it is expected that MD simulation will be a foundation for the multi-scale modeling in sintering process.
-
Citations
Citations to this article as recorded by- Mesoscale modelling of polymer powder densification due to thermal sintering
Amine Bahloul, Issam Doghri, Laurent Adam
Applied Mathematical Modelling.2023; 114: 408. CrossRef - Review of “Integrated Computer-Aided Process Engineering Session in the International Symposium on Innovation in Materials Processing (ISIMP, 26–29 October 2021)”
Hyunjoo Choi, Jungjoon Kim, Pil-Ryung Cha, Hyoung Seop Kim
MATERIALS TRANSACTIONS.2023; 64(10): 2542. CrossRef - Enhancement in electrical conductivity of pastes containing submicron Ag-coated Cu filler with palmitic acid surface modification
Eun Byeol Choi, Jong-Hyun Lee
Applied Surface Science.2017; 415: 67. CrossRef
- Mesoscale modelling of polymer powder densification due to thermal sintering
- [Korean]
- Synthesis of CNT on a Camphene Impregnated Titanium Porous Body by Thermal Chemical Vapor Deposition
- Hogyu Kim, Hye Rim Choi, Jong Min Byun, Myung-Jin Suk, Sung-Tag Oh, Young Do Kim
- J Korean Powder Metall Inst. 2015;22(2):122-128. Published online April 1, 2015
- DOI: https://doi.org/10.4150/KPMI.2015.22.2.122
- 801 View
- 1 Download
- 3 Citations
-
 Abstract
Abstract
 PDF
PDF In this study, titanium(Ti) meshes and porous bodies are employed to synthesize carbon nanotubes(CNTs) using methane(CH4) gas and camphene solution, respectively, by chemical vapor deposition. Camphene is impregnated into Ti porous bodies prior to heating in a furnace. Various microscopic and spectroscopic techniques are utilized to analyze CNTs. It is found that CNTs are more densely and homogeneously populated on the camphene impregnated Ti-porous bodies as compared to CNTs synthesized with methane on Ti-porous bodies. It is elucidated that, when synthesized with methane, few CNTs are formed inside of Ti porous bodies due to methane supply limited by internal structures of Ti porous bodies. Ti-meshes and porous bodies are found to be multi-walled with high degree of structural disorders. These CNTs are expected to be utilized as catalyst supports in catalytic filters and purification systems.
-
Citations
Citations to this article as recorded by- Recent progress in additive manufacturing of porous titanium: From design to applications
Haoxin Song, Chen Wang, Wenzheng Yu, Mingsen Zhang, Jinqiang Shao, Hanwen Liang, Tingting Wu, Xiaoxiao Dong
Journal of Alloys and Compounds.2025; 1026: 180451. CrossRef - Solvent induced surface modifications on hydrogen storage performance of ZnO nanoparticle decorated MWCNTs
Madhavi Konni, Anima S. Dadhich, Saratchandra Babu Mukkamala
Sustainable Energy & Fuels.2018; 2(2): 466. CrossRef - Influence of nickel nanoparticles on hydrogen storage behaviors of MWCNTs
Ye-Ji Han, Soo-Jin Park
Applied Surface Science.2017; 415: 85. CrossRef
- Recent progress in additive manufacturing of porous titanium: From design to applications
TOP
 KPMI
KPMI


 First
First Prev
Prev