Search
- Page Path
- HOME > Search
- [Korean]
- Effect of Fe and Cr on ω Phase Formation in Metastable β-Ti Alloy
- Sun-Young Park, Young-Bum Chun
- J Powder Mater. 2025;32(4):354-360. Published online August 29, 2025
- DOI: https://doi.org/10.4150/jpm.2025.00220

- 1,024 View
- 16 Download
-
 Abstract
Abstract
 PDF
PDF - This study investigated the effects of Fe and Cr contents on ω phase formation and transformation during solution treatment and the subsequent aging process, for which four model alloys with varying Fe and Cr contents but keeping Mo equivalent of ~ 12.6 were prepared by plasma arc melting and fabricated into plates by hot forging followed by hot-rolling. The atherrmal ω phase was observed in all Ti alloys after solution treatment followed by water quenching through XRD and TEM analysis. The largest volume fraction of athermal ω phase is formed in Ti alloy with only Fe 4 wt.% among all Ti alloys, leading to the highest Vickers value due to hardening effect ω phase. It was found that not only Mo equivalent but also each characteristic of β stabilizing elements should be considered to understand a microstructure evolution and mechanical properties.
- [English]
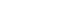
- Recovery of Barium, Nickel, and Titanium Powders from Waste MLCC
- Haein Shin, Kun-Jae Lee
- J Powder Mater. 2024;31(5):374-381. Published online October 31, 2024
- DOI: https://doi.org/10.4150/jpm.2024.00192
- 2,308 View
- 58 Download
-
 Abstract
Abstract
 PDF
PDF - The development of the electronics industry has led to an increased demand for the manufacture of MLCC (Multilayer Ceramic Capacitors), which in turn is expected to result in a rise in MLCC waste. The MLCC contains various metals, notably barium, titanium, and nickel, whose disposal is anticipated to increase correspondingly. Recently, recycling technologies for electronic waste have garnered attention as they address waste management and raw material supply challenges. This paper investigates the recovery of barium, nickel, and titanium from the MLCC by a hydrometallurgical process. Using citric acid, which is an organic acid, the metal inside the MLCC was leached. Additionally, metal materials were recovered through precipitation and complexing processes. As a result, barium and titanium were recovered from the leachate of the waste MLCC, and 93% of the nickel-based powder was recovered. Furthermore, the optimal recovery process conditions for recycling these metal elements were investigated.
- [Korean]
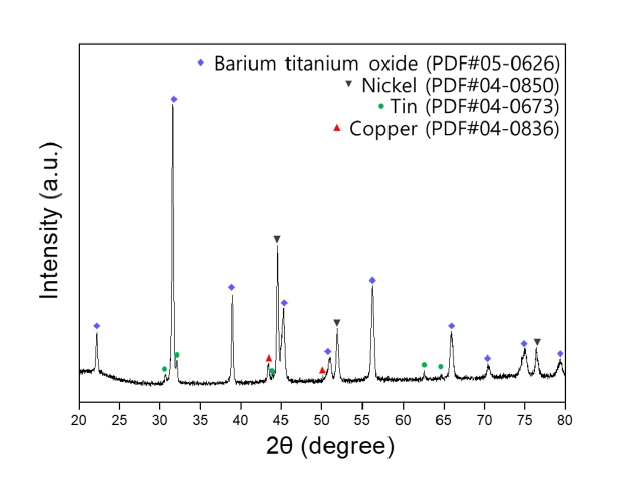
- Fabrication of Ti-Mo Core-shell Powder and Sintering Properties for Application as a Sputtering Target
- Won Hee Lee, Chun Woong Park, Heeyeon Kim, Yuncheol Ha, Jongmin Byun, Young Do Kim
- J Powder Mater. 2024;31(1):43-49. Published online February 28, 2024
- DOI: https://doi.org/10.4150/KPMI.2024.31.1.43
- 1,550 View
- 36 Download
- [Korean]
- The Synthesis of Lithium Lanthanum Titanium Oxide for Solid Electrolyte via Ultrasonic Spray Pyrolysis
- Jaeseok Roh, MinHo Yang, Kun-Jae Lee
- J Powder Mater. 2022;29(6):485-491. Published online December 1, 2022
- DOI: https://doi.org/10.4150/KPMI.2022.29.6.485

- 1,483 View
- 24 Download
-
 Abstract
Abstract
 PDF
PDF Lithium lanthanum titanium oxide (LLTO) is a promising ceramic electrolyte because of its high ionic conductivity at room temperature, low electrical conductivity, and outstanding physical properties. Several routes for the synthesis of bulk LLTO are known, in particular, solid-state synthesis and sol-gel method. However, the extremely low ionic conductivity of LLTO at grain boundaries is one of the major problems for practical applications. To diminish the grain boundary effect, the structure of LLTO is tuned to nanoscale morphology with structures of different dimensionalities (0D spheres, and 1D tubes and wires); this strategy has great potential to enhance the ion conduction by intensifying Li diffusion and minimizing the grain boundary resistance. Therefore, in this work, 0D spherical LLTO is synthesized using ultrasonic spray pyrolysis (USP). The USP method primarily yields spherical particles from the droplets generated by ultrasonic waves passed through several heating zones. LLTO is synthesized using USP, and the effects of each precursor and their mechanisms as well as synthesis parameters are analyzed and discussed to optimize the synthesis. The phase structure of the obtained materials is analyzed using X-ray diffraction, and their morphology and particle size are analyzed using field-emission scanning electron microscopy.
- [Korean]
- Effect of Iron Content on Microstructure and Mechanical Properties of Ti-Mo-Fe P/M Alloys
- HyoWoon Hwang, YongJae Lee, JiHwan Park, Dong-Geun Lee
- J Powder Mater. 2022;29(4):325-331. Published online August 1, 2022
- DOI: https://doi.org/10.4150/KPMI.2022.29.4.325
- 1,207 View
- 6 Download
- 3 Citations
-
 Abstract
Abstract
 PDF
PDF Beta-titanium alloys are used in many industries due to their increased elongation resulting from their BCC structure and low modulus of elasticity. However, there are many limitations to their use due to the high cost of betastabilizer elements. In this study, biocompatible Ti-Mo-Fe beta titanium alloys are designed by replacing costly betastabilizer elements (e.g., Nb, Zr, or Ta) with inexpensive Mo and Fe elements. Additionally, Ti-Mo-Fe alloys designed with different Fe contents are fabricated using powder metallurgy. Fe is a strong, biocompatible beta-stabilizer element and a low-cost alloying element. The mechanical properties of the Ti-Mo-Fe metastable beta titanium alloys are analyzed in relation to the microstructural changes. When the Fe content increases, the tensile strength and elongation decrease due to brittle fracture despite a decreasing pore fraction. It is confirmed that the hardness and tensile strength of Ti-5Mo-2Fe P/M improve to more than 360 Hv and 900 MPa, respectively.
-
Citations
Citations to this article as recorded by- Cost-effective Fabrication of Near β-Ti Alloy via L-PBF: Process Optimization of In-situ Alloying Ti-3Fe
Sehun Kim, Ukju Gim, Taehu Kang, Jongik Lee, Sanghee Jeong, Jimin Han, Bin Lee
Journal of Powder Materials.2025; 32(4): 288. CrossRef - Fabrication of Ti-Mo Core-shell Powder and Sintering Properties for Application as a Sputtering Target
Won Hee Lee, Chun Woong Park, Heeyeon Kim, Yuncheol Ha, Jongmin Byun, Young Do Kim
journal of Korean Powder Metallurgy Institute.2024; 31(1): 43. CrossRef - Effect of Strain Rate on Deformation Behaviors of Ti-12.1Mo -1Fe Metastable Beta Alloy
In Kyeong Jin, Dong-Geun Lee
Korean Journal of Metals and Materials.2023; 61(10): 741. CrossRef
- Cost-effective Fabrication of Near β-Ti Alloy via L-PBF: Process Optimization of In-situ Alloying Ti-3Fe
- [Korean]
- Comparison Study of Compact Titanium Oxide (c-TiO2) Powder Electron Transport Layer Fabrication for Carbon Electrode-based Perovskite Solar Cells
- Chae Young Woo, Hyung Woo Lee
- J Powder Mater. 2022;29(4):297-302. Published online August 1, 2022
- DOI: https://doi.org/10.4150/KPMI.2022.29.4.297

- 827 View
- 7 Download
-
 Abstract
Abstract
 PDF
PDF This study compares the characteristics of a compact TiO2 (c-TiO2) powdery film, which is used as the electron transport layer (ETL) of perovskite solar cells, based on the manufacturing method. Additionally, its efficiency is measured by applying it to a carbon electrode solar cell. Spin-coating and spray methods are compared, and spraybased c-TiO2 exhibits superior optical properties. Furthermore, surface analysis by scanning electron microscopy (SEM) and atomic force microscopy (AFM) exhibits the excellent surface properties of spray-based TiO2. The photoelectric conversion efficiency (PCE) is 14.31% when applied to planar perovskite solar cells based on metal electrodes. Finally, carbon nanotube (CNT) film electrode-based solar cells exhibits a 76% PCE compared with that of metal electrodebased solar cells, providing the possibility of commercialization.
- [Korean]
- Current Status of Titanium Smelting Technology for Powder Metallurgy
- Ho-Sang Sohn
- J Korean Powder Metall Inst. 2021;28(2):164-172. Published online April 1, 2021
- DOI: https://doi.org/10.4150/KPMI.2021.28.2.164

- 836 View
- 8 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
PDF Titanium is the ninth most abundant element in the Earth’s crust and is the fourth most abundant structural metal after aluminum, iron, and magnesium. It exhibits a higher specific strength than steel along with an excellent corrosion resistance, highlighting the promising potential of titanium as a structural metal. However, titanium is difficult to extract from its ore and is classified as a rare metal, despite its abundance. Therefore, the production of titanium is exceedingly low compared to that of common metals. Titanium is conventionally produced as a sponge by the Kroll process. For powder metallurgy (PM), hydrogenation-dehydrogenation (HDH) of the titanium sponge or gas atomization of the titanium bulk is required. Therefore, numerous studies have been conducted on smelting, which replaces the Kroll process and produces powder that can be used directly for PM. In this review, the Kroll process and new smelting technologies of titanium for PM, such as metallothermic, electrolytic, and hydrogen reduction of TiCl4 and TiO2 are discussed.
-
Citations
Citations to this article as recorded by- Enhancing corrosion resistance of Ti-based amorphous alloy powders via misch metal addition
Yeon Joo Lee, Hyokyung Sung, Jae Bok Seol, Kisub Cho, Hwi Jun Kim, Hyunjoo Choi
Powder Metallurgy.2025; 68(3): 230. CrossRef
- Enhancing corrosion resistance of Ti-based amorphous alloy powders via misch metal addition
- [Korean]
- Microstructure and Mechanical Property of Ti-Mn-Cu Alloys with Magnetic Pulsed Compaction
- Ye Jun Yun, Chun Woong Park, Won June Choi, Jongmin Byun
- J Korean Powder Metall Inst. 2021;28(1):20-24. Published online February 1, 2021
- DOI: https://doi.org/10.4150/KPMI.2021.28.1.20
- 833 View
- 6 Download
-
 Abstract
Abstract
 PDF
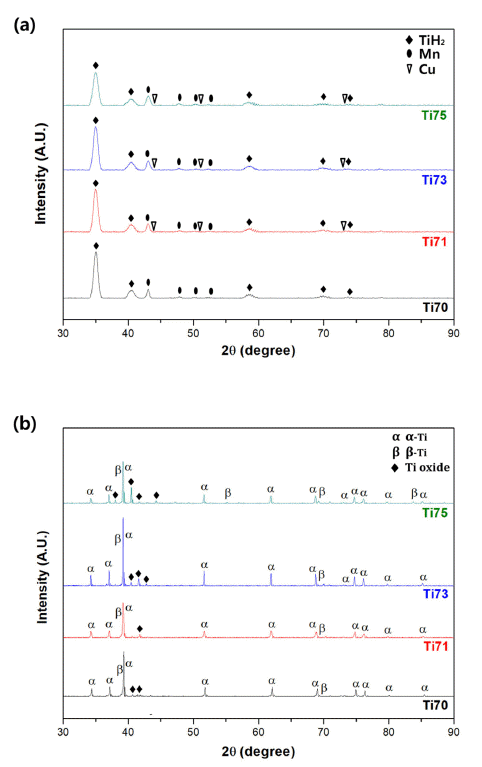
PDF Ti-based alloys are widely used in biomaterials owing to their excellent biocompatibility. In this study, Ti- Mn-Cu alloys are prepared by high-energy ball milling, magnetic pulsed compaction, and pressureless sintering. The microstructure and microhardness of the Ti-Mn-Cu alloys with variation of the Cu addition and compaction pressure are analyzed. The correlation between the composition, compaction pressure, and density is investigated by measuring the green density and sintered density for samples with different compositions, subjected to various compaction pressures. For all compositions, it is confirmed that the green density increases proportionally as the compaction pressure increases, but the sintered density decreases owing to gas formation from the pyrolysis of TiH2 powders and reduction of oxides on the surface of the starting powders during the sintering process. In addition, an increase in the amount of Cu addition changes the volume fractions of the α-Ti and β-Ti phases, and the microstructure of the alloys with different compositions also changes. It is demonstrated that these changes in the phase volume fraction and microstructure are closely related to the mechanical properties of the Ti-Mn-Cu alloys.
- [Korean]
- A Study on the Preparation and Growth Mechanism of Titanium Dioxide using Organic-Inorganic Hybrid Titanium Complex
- Yubin Kang, Jin-Ju Choi, Nam Hun Kwon, Dae-Guen Kim, Kun-Jae Lee
- J Korean Powder Metall Inst. 2019;26(6):487-492. Published online December 1, 2019
- DOI: https://doi.org/10.4150/KPMI.2019.26.6.487
- 1,178 View
- 15 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
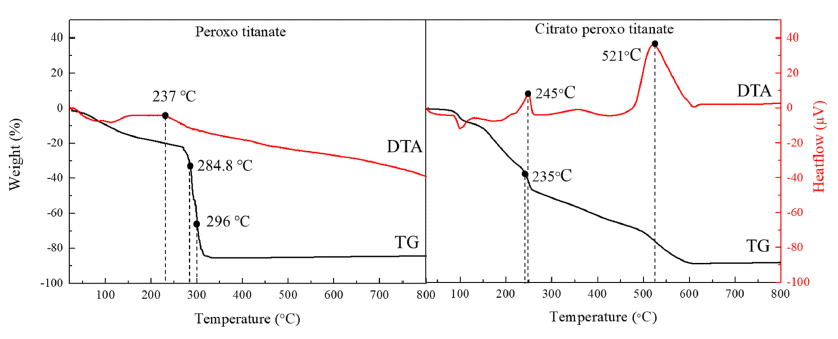
PDF Titanium dioxide (TiO2) is a typical inorganic material that has an excellent photocatalytic property and a high refractive index. It is used in water/air purifiers, solar cells, white pigments, refractory materials, semiconductors, etc.; its demand is continuously increasing. In this study, anatase and rutile phase titanium dioxide is prepared using hydroxyl and carboxyl; the titanium complex and its mechanism are investigated. As a result of analyzing the phase transition characteristics by a heat treatment temperature using a titanium complex having a hydroxyl group and a carboxyl group, it is confirmed that the material properties were different from each other and that the anatase and rutile phase contents can be controlled. The titanium complexes prepared in this study show different characteristics from the titania-formation temperatures of the known anatase and rutile phases. It is inferred that this is due to the change of electrostatic adsorption behavior due to the complexing function of the oxygen sharing point, which crystals of the TiO6 structure share.
-
Citations
Citations to this article as recorded by- Thermal Stability and Weight Reduction of Al0.75V2.82CrZr Refractory High Entropy Alloy Prepared Via Mechanical Alloying
Minsu Kim, Hansung Lee, Byungmin Ahn
journal of Korean Powder Metallurgy Institute.2023; 30(6): 478. CrossRef
- Thermal Stability and Weight Reduction of Al0.75V2.82CrZr Refractory High Entropy Alloy Prepared Via Mechanical Alloying
- [Korean]
- Joint Properties of Stainless Steel and Titanium Alloys Additive Manufactured on Medium Entropy Alloys
- Chan Woong Park, Nana Kwabena Adomako, Min Gyu Lee, Jeoung Han Kim
- J Korean Powder Metall Inst. 2019;26(4):319-326. Published online August 1, 2019
- DOI: https://doi.org/10.4150/KPMI.2019.26.4.319

- 744 View
- 5 Download
-
 Abstract
Abstract
 PDF
PDF Additive manufacturing (AM) is a highly innovative method for joining dissimilar materials for industrial applications. In the present work, AM of STS630 and Ti-6Al-4V powder alloys on medium entropy alloys (MEAs) NiCrCo and NiCrCoMn is studied. The STS630 and Ti64 powders are deposited on the MEAs. Joint delamination and cracks are observed after the deposition of Ti64 on the MEAs, whereas the deposition of STS630 on the MEAs is successful, without any cracks and joint delamination. The microstructure around the fusion zone interface is characterized by scanning electron microscopy and X-ray diffraction. Intermetallic compounds are formed at the interfacial regions of MEA-Ti64 samples. In addition, Vicker’s hardness value increased dramatically at the joint interface between MEAs and Ti-6Al-4V compared to that between MEAs and STS630. This result is attributed to the brittle nature of the joint, which can lead to a decrease in the joint strength.
- [Korean]
- Study on Manufacture of High Purity TiCl4 and Synthesis of High Purity Ti Powders
- Jieun Lee, Jin-Ho Yoon, Chan Gi Lee
- J Korean Powder Metall Inst. 2019;26(4):282-289. Published online August 1, 2019
- DOI: https://doi.org/10.4150/KPMI.2019.26.4.282
- 1,436 View
- 27 Download
-
 Abstract
Abstract
 PDF
PDF Ti has received considerable attention for aerospace, vehicle, and semiconductor industry applications because of its acid-resistant nature, low density, and high mechanical strength. A common precursor used for preparing Ti materials is TiCl4. To prepare high-purity TiCl4, a process based on the removal of VOCl3 has been widely applied. However, VOCl3 removal by distillation and condensation is difficult because of the similar physical properties of TiCl4 and VOCl3. To circumvent this problem, in this study, we have developed a process for VOCl3 removal using Cu powder and mineral oil as purifying agents. The effects of reaction time and temperature, and ratio of purifying agents on the VOCl3 removal efficiency are investigated by chemical and structural measurements. Clear TiCl4 is obtained after the removal of VOCl3. Notably, complete removal of VOCl3 is achieved with 2.0 wt% of mineral oil. Moreover, the refined TiCl4 is used as a precursor for the synthesis of Ti powder. Ti powder is fabricated by a thermal reduction process at 1,100ºC using an H2-Ar gas mixture. The average size of the Ti powder particles is in the range of 1-3 μm.
- [Korean]
- Microstructure and Mechanical Properties of Oxide Dispersion Strengthened alloy Based on Commercially Pure Titanium
- Taesung Park, Jeoung Han Kim
- J Korean Powder Metall Inst. 2018;25(4):327-330. Published online August 1, 2018
- DOI: https://doi.org/10.4150/KPMI.2018.25.4.327

- 1,006 View
- 9 Download
- 4 Citations
-
 Abstract
Abstract
 PDF
PDF This study is conducted as a preliminary research to verify the feasibility of Ti-based Oxide dispersion strengthened (ODS) alloy. Pure-Ti powder is mixed with Y2O3 powder and subsequently, mechanically alloyed at -150°C. The Ti-based ODS powder is hot-isostatically pressed and subsequently hot-rolled for recrystallization. The microstructure consists of elongated grains and Y excess fine particles. The oxide particle size is larger than that of the typical Febased ODS steel. Tensile test shows that the tensile ductility is approximately 25%, while the strength is significantly higher than that of pure Ti. The high-temperature hardness of the Ti-ODS alloy is also significantly higher than that of pure Ti at all temperatures, while being lower than that of Ti-6Al-4V. The dimple structure is well developed, and no evidence of cleavage fracture surface is observed in the fracture surface of the tensile specimen.
-
Citations
Citations to this article as recorded by- Additive manufacturing of Ti-6Al-4V based oxide dispersion strengthened alloy using in-situ oxide-dispersed powders and bound metal deposition
Woo Hyeok Kim, Raj Narayan Hajra, Hyung-Ki Park, Jung-Yeul Yun, Jeoung Han Kim
Journal of Alloys and Compounds.2026; 1050: 185574. CrossRef - Experimental and Numerical Evaluation of Rockwell Hardness at High Temperatures
NamSeok Lee
Journal of the Korean Society of Manufacturing Technology Engineers.2025; 34(3): 165. CrossRef - Spheroidization of Pure-vanadium Powder using Radio Frequency Thermal Plasma Process
Nana Kwabena Adomako, Seungmin Yang, Min Gyu Lee, N. S. Reddy, Jeoung-Han Kim
Journal of Korean Powder Metallurgy Institute.2019; 26(4): 305. CrossRef - Microstructure and Mechanical Properties of Friction-Welded Alloy 718 and SNCRW Stainless Steel After Post-Weld Heat-Treatment
Jeoung Han Kim, Nam-Yong Kim, Yu Sik Kong, Nho Kwang Park
Journal of Welding and Joining.2019; 37(4): 313. CrossRef
- Additive manufacturing of Ti-6Al-4V based oxide dispersion strengthened alloy using in-situ oxide-dispersed powders and bound metal deposition
- [Korean]
- Photocatalysis of TiO2/WO3 Composites Synthesized by Ball Milling
- Su-Yeol Yu, Chunghee Nam
- J Korean Powder Metall Inst. 2018;25(4):316-321. Published online August 1, 2018
- DOI: https://doi.org/10.4150/KPMI.2018.25.4.316

- 798 View
- 3 Download
-
 Abstract
Abstract
 PDF
PDF Composites of P25 TiO2 and hexagonal WO3 nanorods are synthesized through ball-milling in order to study photocatalytic properties. Various composites of TiO2/WO3 are prepared by controlling the weight percentages (wt%) of WO3, in the range of 1–30 wt%, and milling time to investigate the effects of the composition ratio on the photocatalytic properties. Scanning electron microscopy, x-ray diffraction, and transmission electron microscopy are performed to characterize the structure, shape and size of the synthesized composites of TiO2/WO3. Methylene blue is used as a test dye to analyze the photocatalytic properties of the synthesized composite material. The photocatalytic activity shows that the decomposition efficiency of the dye due to the photocatalytic effect is the highest in the TiO2/WO3 (3 wt%) composite, and the catalytic efficiency decreases sharply when the amount of WO3 is further increased. As the amount of WO3 added increases, dye-removal by adsorption occurs during centrifugation, instead of the decomposition of dyes by photocatalysts. Finally, TiO2/WO3 (3 wt%) composites are synthesized with various milling times. Experimental results show that the milling time has the best catalytic efficiency at 30 min, after which it gradually decreases. There is no significant change after 1 hour.
- [Korean]
- Photocatalytic activity of rutile TiO2 powders coupled with anatase TiO2 nanoparticles using surfactant
- Jong Min Byun, Chun Woong Park, Young In Kim, Young Do Kim
- J Korean Powder Metall Inst. 2018;25(3):257-262. Published online June 1, 2018
- DOI: https://doi.org/10.4150/KPMI.2018.25.3.257

- 1,161 View
- 9 Download
- 2 Citations
-
 Abstract
Abstract
 PDF
PDF The coupling of two semiconducting materials is an efficient method to improve photocatalytic activity via the suppression of recombination of electron-hole pairs. In particular, the coupling between two different phases of TiO2, i.e., anatase and rutile, is particularly attractive for photocatalytic activity improvement of rutile TiO2 because these coupled TiO2 powders can retain the benefits of TiO2, one of the best photocatalysts. In this study, anatase TiO2 nanoparticles are synthesized and coupled on the surface of rutile TiO2 powders using a microemulsion method and heat treatment. Triton X-100, as a surfactant, is used to suppress the aggregation of anatase TiO2 nanoparticles and disperse anatase TiO2 nanoparticles uniformly on the surface of rutile TiO2 powders. Rutile TiO2 powders coupled with anatase TiO2 nanoparticles are successfully prepared. Additionally, we compare the photocatalytic activity of these rutile-anatase coupled TiO2 powders under ultraviolet (UV) light and demonstrate that the reason for the improvement of photocatalytic activity is microstructural.
-
Citations
Citations to this article as recorded by- Refractory Metal Oxide–Doped Titanate Nanotubes: Synthesis and Photocatalytic Activity under UV/Visible Light Range
Min-Sang Kim, Hyun-Joo Choi, Tohru Sekino, Young-Do Kim, Se-Hoon Kim
Catalysts.2021; 11(8): 987. CrossRef - Effect of Surfactant on the Dispersion Stability of Slurry for Semiconductor Silicon CMP
Hye Won Yun, Doyeon Kim, Do Hyung Han, Dong Wan Kim, Woo-Byoung Kim
Journal of Korean Powder Metallurgy Institute.2018; 25(5): 395. CrossRef
- Refractory Metal Oxide–Doped Titanate Nanotubes: Synthesis and Photocatalytic Activity under UV/Visible Light Range
- [Korean]
- Fabrication of TiC powder by carburization of TiH2 powder
- Hun-Seok Lee, Hyang-Im Seo, Young-Seon Lee, Dong-Jun Lee, Jei-Pil Wang, Dong-Won Lee
- J Korean Powder Metall Inst. 2017;24(1):29-33. Published online February 1, 2017
- DOI: https://doi.org/10.4150/KPMI.2017.24.1.29
- 694 View
- 2 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
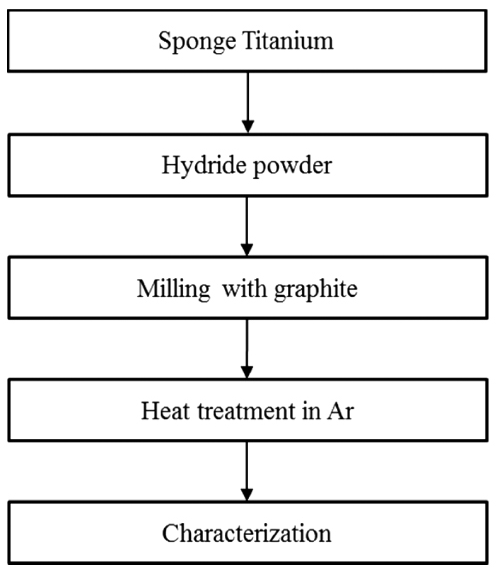
PDF Titanium carbide (TiC) powders are successfully synthesized by carburization of titanium hydride (TiH2) powders. The TiH2 powders with size lower than 45 μm (-325 Mesh) are optimally produced by the hydrogenation process, and are mixed with graphite powder by ball milling. The mixtures are then heat-treated in an Ar atmosphere at 800-1200oC for carburization to occur. It has been experimentally and thermodynamically determined that the dehydrogenation, “TiH2 = Ti + H2”, and carburization, “Ti + C = TiC”, occur simultaneously over the reaction temperature range. The unreacted graphite content (free carbon) in each product is precisely measured by acid dissolution and by the filtering method, and it is possible to conclude that the maximal carbon stoichiometry of TiC0.94 is accomplished at 1200°C.
-
Citations
Citations to this article as recorded by- Pre-treatments of initial materials for controlling synthesized TaC characteristics in the SHS process
Jae Jin Sim, Sang Hoon Choi, Ji Hwan Park, Il Kyu Park, Jae Hong Lim, Kyoung Tae Park
journal of Korean Powder Metallurgy Institute.2018; 25(3): 251. CrossRef
- Pre-treatments of initial materials for controlling synthesized TaC characteristics in the SHS process
- [English]
- Fabrication of Sintered Compact of Fe-TiB2 Composites by Pressureless Sintering of (FeB+TiH2) Powder Mixture
- Xuan-Khoa Huynh, Ji Soon Kim
- J Korean Powder Metall Inst. 2016;23(4):282-286. Published online August 1, 2016
- DOI: https://doi.org/10.4150/KPMI.2016.23.4.282
- 1,034 View
- 1 Download
- 3 Citations
-
 Abstract
Abstract
 PDF
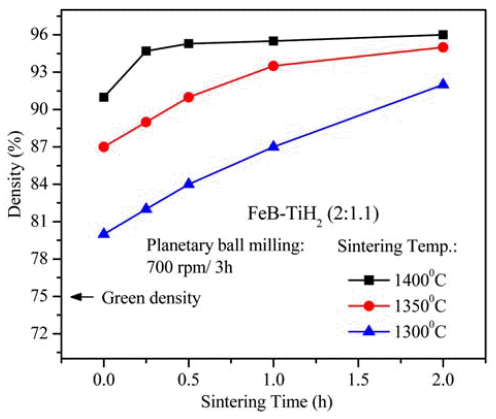
PDF A sintered body of TiB2-reinforced iron matrix composite (Fe-TiB2) is fabricated by pressureless-sintering of a mixture of titanium hydride (TiH2) and iron boride (FeB) powders. The powder mixture is prepared in a planetary ball-mill at 700 rpm for 3 h and then pressurelessly sintered at 1300, 1350 and 1400°C for 0-2 h. The optimal sintering temperature for high densities (above 95% relative density) is between 1350 and 1400°C, where the holding time can be varied from 0.25 to 2 h. A maximum relative density of 96.0% is obtained from the (FeB+TiH2) powder compacts sintered at 1400°C for 2 h. Sintered compacts have two main phases of Fe and TiB2 along with traces of TiB, which seems to be formed through the reaction of TiB2 formed at lower temperatures during the heating stage with the excess Ti that is intentionally added to complete the reaction for TiB2 formation. Nearly fully densified sintered compacts show a homogeneous microstructure composed of fine TiB2 particulates with submicron sizes and an Fe-matrix. A maximum hardness of 71.2 HRC is obtained from the specimen sintered at 1400°C for 0.5 h, which is nearly equivalent to the HRC of conventional WC-Co hardmetals containing 20 wt% Co.
-
Citations
Citations to this article as recorded by- Optimizing the Microstructure and Properties of Fe–Ni–Cu–Mo–C Sintered Steel by TiB2
Zenglin Liu, Yankang Wang, Weilong Lu, Feng Liu, Wei Han, Wuqiang He
Science of Advanced Materials.2024; 16(6): 707. CrossRef - Effect of Ce Addition on the As-Cast and As-Forged Microstructure of Fe-TiB2 Composites
Lin Zhang, Jianwen Gao, Minghao Huang, Engang Wang
JOM.2019; 71(11): 4144. CrossRef - Microstructure, mechanical, and tribological properties of pressureless sintered and spark plasma sintered Fe TiB2 nanocomposites
Hak-Rae Cho, Ji-Soon Kim, Koo-Hyun Chung
Tribology International.2019; 131: 83. CrossRef
- Optimizing the Microstructure and Properties of Fe–Ni–Cu–Mo–C Sintered Steel by TiB2
- [Korean]
- Fabrication of Ti Porous body with Improved Specific Surface Area by Synthesis of CNTs
- Hye Rim Choi, Jong Min Byun, Myung-Jin Suk, Sung-Tag Oh, Young Do Kim
- J Korean Powder Metall Inst. 2016;23(3):235-239. Published online June 1, 2016
- DOI: https://doi.org/10.4150/KPMI.2016.23.3.235

- 496 View
- 0 Download
-
 Abstract
Abstract
 PDF
PDF This study is performed to fabricate a Ti porous body by freeze drying process using titanium hydride (TiH2) powder and camphene. Then, the Ti porous body is employed to synthesize carbon nanotubes (CNTs) using thermal catalytic chemical vapor deposition (CCVD) with Fe catalyst and methane (CH4) gas to increase the specific surface area. The synthesized Ti porous body has 100 μm-sized macropores and 10-30 μm-sized micropores. The synthesized CNTs have random directions and are entangled with adjacent CNTs. The CNTs have a bamboo-like structure, and their average diameter is about 50 nm. The Fe nano-particles observed at the tip of the CNTs indicate that the tip growth model is applicable. The specific surface area of the CNT-coated Ti porous body is about 20 times larger than that of the raw Ti porous body. These CNT-coated Ti porous bodies are expected to be used as filters or catalyst supports.
- [Korean]
- The Preparation of Dye-Sensitized Solar Cell Paste Used the Peroxo Titanium Complex and Characteristics by Annealing Temperature
- Hyunsu Park, Soyeong Joo, Joon-Phil Choi, Woo-Byoung Kim
- J Korean Powder Metall Inst. 2015;22(6):396-402. Published online December 1, 2015
- DOI: https://doi.org/10.4150/KPMI.2015.22.6.396

- 1,025 View
- 6 Download
- 5 Citations
-
 Abstract
Abstract
 PDF
PDF The organic binder-free paste for dye-sensitized solar cell (DSSC) has been investigated using peroxo titanium complex. The crystal structure of TiO2 nanoparticles, morphology of TiO2 film and electrical properties are analyzed by X-Ray Diffraction (XRD), Scanning Electron Microscopy (SEM), Electrochemical Impedance Spectra (EIS), and solar simulator. The synthesized TiO2 nanopowders by the peroxo titanium complex at 150, 300, 400°C, and 450°C have anatase phase and average crystal sizes are calculated to be 4.2, 13.7, 16.9, and 20.9 nm, respectively. The DSSC prepared by the peroxo titanium complex binder have higher Voc and lower Jsc values than that of the organic binder. It can be attributed to improvement of sintering properties of TCO/TiO2 and TiO2/TiO2 interface and to formation of agglomerate by the nanoparticles. As a result, we have investigated the organic binder-free paste and 3.178% conversion efficiency of the DSSC at 450°C.
-
Citations
Citations to this article as recorded by- Development of Eco-Friendly Ag Embedded Peroxo Titanium Complex Solution Based Thin Film and Electrical Behaviors of Resistive Random Access Memory
Won Jin Kim, Jinho Lee, Ryun Na Kim, Donghee Lee, Woo-Byoung Kim
Korean Journal of Materials Research.2024; 34(3): 152. CrossRef - Development of eco-friendly thin film manufacturing process using poeroxo titanium complex solution and potential for resistive random access memory
Jinho Lee, Ryun Na Kim, Kee-Ryung Park, Woo-Byoung Kim
Applied Surface Science.2021; 562: 150170. CrossRef - Preparation of ultra-thin TiO2 shell by peroxo titanium complex (PTC) solution-based green surface modification, and photocatalytic activity of homo-core/shell TiO2
Jinho Lee, Jiyong Hwang, Hyunsu Park, Tohru Sekino, Woo-Byoung Kim
Applied Surface Science.2021; 540: 148399. CrossRef - Effects of Annealing Temperature on the Crystal Structure, Morphology, and Optical Properties of Peroxo-Titanate Nanotubes Prepared by Peroxo-Titanium Complex Ion
Hyunsu Park, Tomoyo Goto, Sunghun Cho, Soo Wohn Lee, Masato Kakihana, Tohru Sekino
Nanomaterials.2020; 10(7): 1331. CrossRef - Study on thermal behavior of Ammonium Hexafluofide Titanate for Synthesis of TiO2 Powders
Duk-Hee Lee, Jae-Ryang Park, Chan-Gi Lee, Kyung-Soo Park, Hyeon-Mo Kim
Journal of Korean Powder Metallurgy Institute.2016; 23(5): 353. CrossRef
- Development of Eco-Friendly Ag Embedded Peroxo Titanium Complex Solution Based Thin Film and Electrical Behaviors of Resistive Random Access Memory
- [Korean]
- Synthesis of CNT on a Camphene Impregnated Titanium Porous Body by Thermal Chemical Vapor Deposition
- Hogyu Kim, Hye Rim Choi, Jong Min Byun, Myung-Jin Suk, Sung-Tag Oh, Young Do Kim
- J Korean Powder Metall Inst. 2015;22(2):122-128. Published online April 1, 2015
- DOI: https://doi.org/10.4150/KPMI.2015.22.2.122
- 805 View
- 1 Download
- 3 Citations
-
 Abstract
Abstract
 PDF
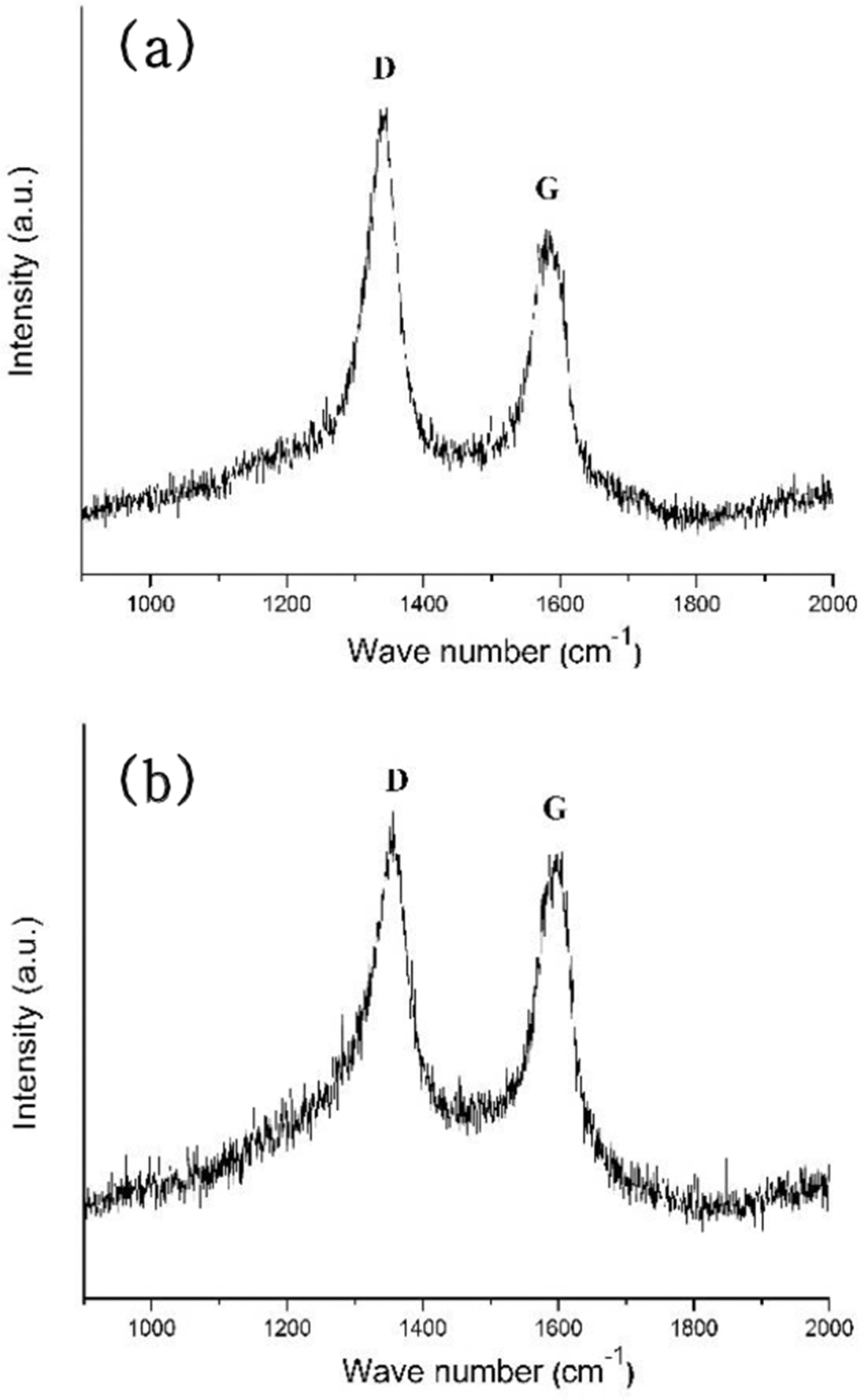
PDF In this study, titanium(Ti) meshes and porous bodies are employed to synthesize carbon nanotubes(CNTs) using methane(CH4) gas and camphene solution, respectively, by chemical vapor deposition. Camphene is impregnated into Ti porous bodies prior to heating in a furnace. Various microscopic and spectroscopic techniques are utilized to analyze CNTs. It is found that CNTs are more densely and homogeneously populated on the camphene impregnated Ti-porous bodies as compared to CNTs synthesized with methane on Ti-porous bodies. It is elucidated that, when synthesized with methane, few CNTs are formed inside of Ti porous bodies due to methane supply limited by internal structures of Ti porous bodies. Ti-meshes and porous bodies are found to be multi-walled with high degree of structural disorders. These CNTs are expected to be utilized as catalyst supports in catalytic filters and purification systems.
-
Citations
Citations to this article as recorded by- Recent progress in additive manufacturing of porous titanium: From design to applications
Haoxin Song, Chen Wang, Wenzheng Yu, Mingsen Zhang, Jinqiang Shao, Hanwen Liang, Tingting Wu, Xiaoxiao Dong
Journal of Alloys and Compounds.2025; 1026: 180451. CrossRef - Solvent induced surface modifications on hydrogen storage performance of ZnO nanoparticle decorated MWCNTs
Madhavi Konni, Anima S. Dadhich, Saratchandra Babu Mukkamala
Sustainable Energy & Fuels.2018; 2(2): 466. CrossRef - Influence of nickel nanoparticles on hydrogen storage behaviors of MWCNTs
Ye-Ji Han, Soo-Jin Park
Applied Surface Science.2017; 415: 85. CrossRef
- Recent progress in additive manufacturing of porous titanium: From design to applications
- [Korean]
- CNT Growth Behavior on Ti Substrate by Catalytic CVD Process with Temperature Gradient in Tube Furnace
- Ju Hyuk Park, Jong Min Byun, Hyung Soo Kim, Myung-Jin Suk, Sung-Tag Oh, Young Do Kim
- J Korean Powder Metall Inst. 2014;21(5):371-376. Published online October 1, 2014
- DOI: https://doi.org/10.4150/KPMI.2014.21.5.371
- 1,074 View
- 2 Download
- 2 Citations
-
 Abstract
Abstract
 PDF
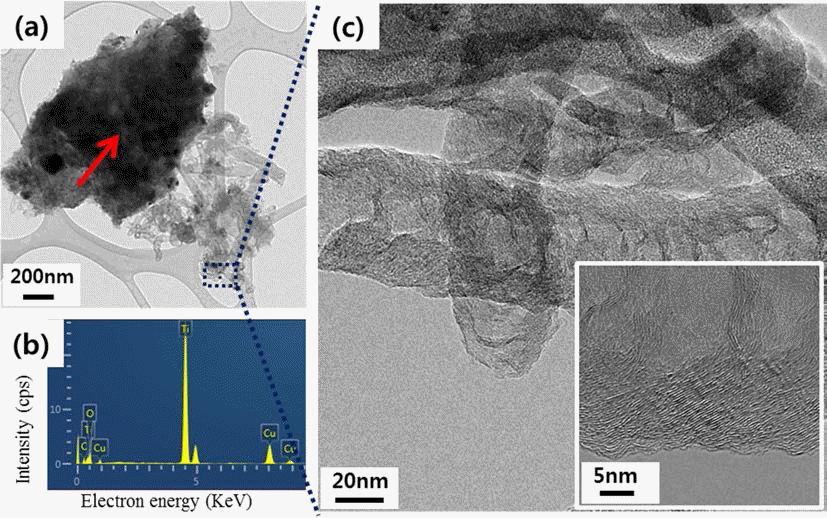
PDF In this study, modified catalytic chemical vapor deposition (CCVD) method was applied to control the CNTs (carbon nanotubes) growth. Since titanium (Ti) substrate and iron (Fe) catalysts react one another and form a new phase (Fe2TiO5) above 700°C, the decrease of CNT yield above 800°C where methane gas decomposes is inevitable under common CCVD method. Therefore, we synthesized CNTs on the Ti substrate by dividing the tube furnace into two sections (left and right) and heating them to different temperatures each. The reactant gas flew through from the end of the right tube furnace while the Ti substrate was placed in the center of the left tube furnace. When the CNT growth temperature was set 700/950°C (left/right), CNTs with high yield were observed. Also, by examining the micro-structure of CNTs of 700/950°C, it was confirmed that CNTs show the bamboo-like structure.
-
Citations
Citations to this article as recorded by- Fabrication of Ti Porous body with Improved Specific Surface Area by Synthesis of CNTs
Hye Rim Choi, Jong Min Byun, Myung-Jin Suk, Sung-Tag Oh, Young Do Kim
Journal of Korean Powder Metallurgy Institute.2016; 23(3): 235. CrossRef - Synthesis of CNT on a Camphene Impregnated Titanium Porous Body by Thermal Chemical Vapor Deposition
Hogyu Kim, Hye Rim Choi, Jong Min Byun, Myung-Jin Suk, Sung-Tag Oh, Young Do Kim
Journal of Korean Powder Metallurgy Institute.2015; 22(2): 122. CrossRef
- Fabrication of Ti Porous body with Improved Specific Surface Area by Synthesis of CNTs
- [Korean]
- Hot Deformation Behavior and Microstructural Evolution of Powder Metallurgy Ti-6Al-4V Alloy
- Youngmoo Kim, Young-Beom Song, Sung Ho Lee, Young-Sam Kwon
- J Korean Powder Metall Inst. 2014;21(4):277-285. Published online August 1, 2014
- DOI: https://doi.org/10.4150/KPMI.2014.21.4.277

- 1,055 View
- 9 Download
- 3 Citations
-
 Abstract
Abstract
 PDF
PDF The effects of processing parameters on the flow behavior and microstructures were investigated in hot compression of powder metallurgy (P/M) Ti-6Al-4V alloy. The alloy was fabricated by a blended elemental (B/E) approach and it exhibited lamellar α+β microstructure. The hot compression tests were performed in the range of temperature 800-1000°C with 50°C intervals, strain rate 10−4-10 s−1, and strain up to 0.5. At 800-950°C, continuous flow softening after a peak stress was observed with strain rates lower than 0.1 s−1. At strain rates higher than 1 s−1, rapid drop in flow stress with strain hardening or broad oscillations was recorded. The processing map of P/M Ti-6Al-4V was designed based on the compression test and revealed the peak efficiency at 850°C and 0.001 s−1. As the processing temperature increased, the volume fraction of β phase was increased. In addition, below 950°C, the globularization of phase at the slower strain rate and kinking microstructures were found. Based on these data, the preferred working condition of the alloy may be in the range of 850-950°C and strain rate of 0.001-0.01 s−1.
-
Citations
Citations to this article as recorded by- Microstructure control and dynamic recrystallization behavior analysis in hot forging of metastable beta Ti-5Mo-4Fe alloy
In-Kyeong Jin, Jae-Gwan Lee, Yong-Jae Lee, Dong-Geun Lee
Journal of Alloys and Compounds.2025; 1010: 178125. CrossRef - High Temperature Deformation and Microstructural Evolution of Homogenized AA 2026 Alloy
HyeonWoo Kang, SooBin Kim, ByoungLok Jang, HeeKook Kim
Korean Journal of Metals and Materials.2023; 61(5): 338. CrossRef - Effect of Fe Content on the Microstructure and Mechanical Properties of Ti-Al-Mo-V-Cr-Fe Alloys
K.C. Bae, J.J. Oak, Y.H. Kim, Y.H. Park
Archives of Metallurgy and Materials.2017; 62(2): 1105. CrossRef
- Microstructure control and dynamic recrystallization behavior analysis in hot forging of metastable beta Ti-5Mo-4Fe alloy
- [Korean]
- Synthesis of Pt@TiO2 Nano-composite via Photochemical Reduction Method
- Ji Young Kim, Jong Min Byun, Jin Woo Kim, Young Do Kim
- J Korean Powder Metall Inst. 2014;21(2):119-123. Published online April 1, 2014
- DOI: https://doi.org/10.4150/KPMI.2014.21.2.119

- 864 View
- 3 Download
- 2 Citations
-
 Abstract
Abstract
 PDF
PDF Pt has been widely used as catalyst for fuel cell and exhausted gas clean systems due to its high catalytic activity. Recently, there have been researches on fabricating composite materials of Pt as a method of reducing the amount of Pt due to its high price. One of the approaches for saving Pt used as catalyst is a core shell structure consisting of Pt layer on the core of the non-noble metal. In this study, the synthesis of Pt shell was conducted on the surface of TiO2 particle, a non-noble material, by applying ultraviolet (UV) irradiation. Anatase TiO2 particles with the average size of 20~30 nm were immersed in the ethanol dissolved with Pt precursor of H2PtCl6∙6H2O and exposed to UV irradiation with the wavelength of 365 nm. It was confirmed that Pt nano-particles were formed on the surface of TiO2 particles by photochemical reduction of Pt ion from the solution. The morphology of the synthesized Pt@TiO2 nano-composite was examined by TEM (Transmission Electron Microscopy).
-
Citations
Citations to this article as recorded by- Photocatalytic activity of rutile TiO2 powders coupled with anatase TiO2 nanoparticles using surfactant
Jong Min Byun, Chun Woong Park, Young In Kim, Young Do Kim
journal of Korean Powder Metallurgy Institute.2018; 25(3): 257. CrossRef - Synthesis and Photo Catalytic Activity of 10 wt%, 20 wt%Li-TiO2 Composite Powders
Hyeong-Chul Kim, Jae-Kil Han
Journal of Korean Powder Metallurgy Institute.2016; 23(1): 33. CrossRef
- Photocatalytic activity of rutile TiO2 powders coupled with anatase TiO2 nanoparticles using surfactant
- [English]
- Advanced PM Processes for Medical Technologies
- Frank Petzoldt, Vera Friederici, Philipp Imgrund, Claus Aumund-Kopp
- J Korean Powder Metall Inst. 2014;21(1):1-6. Published online February 1, 2014
- DOI: https://doi.org/10.4150/KPMI.2014.21.1.1

- 1,220 View
- 5 Download
- 2 Citations
-
 Abstract
Abstract
 PDF
PDF Medical technologies are gaining in importance because of scientific and technical progress in medicine and the increasing average lifetime of people. This has opened up a huge market for medical devices, where complex-shaped metallic parts made from biocompatible materials are in great demand. Today many of these components are already being manufactured by powder metallurgy technologies. This includes mass production of standard products and also customized components. In this paper some aspects related to metal injection molding of Ti and its alloys as well as modifications of microstructure and surface finish were discussed. The process chain of additive manufacturing (AM) was described and the current state of the art of AM processes like Selective Laser Melting and electron beam melting for medical applications was presented.
-
Citations
Citations to this article as recorded by- Enhancing corrosion resistance of Ti-based amorphous alloy powders via misch metal addition
Yeon Joo Lee, Hyokyung Sung, Jae Bok Seol, Kisub Cho, Hwi Jun Kim, Hyunjoo Choi
Powder Metallurgy.2025; 68(3): 230. CrossRef - Spontaneous Formation of Titanium Nitride on the Surface of a Ti Rod Induced by Electro-Discharge-Heat-Treatment in an N2 Atmosphere
W.H. Lee, Y.H. Yoon, Y.H. Kim, Y.K. Lee, J.Y. Kim, S.Y. Chang
Archives of Metallurgy and Materials.2017; 62(2): 1281. CrossRef
- Enhancing corrosion resistance of Ti-based amorphous alloy powders via misch metal addition
TOP
 KPMI
KPMI


 First
First Prev
Prev