Articles
- Page Path
- HOME > J Powder Mater > Volume 31(4); 2024 > Article
-
Critical Review
- A Review of Inorganic Solid Electrolytes for All-Solid-State Lithium Batteries: Challenges and Progress
- Seul Ki Choi1, Jaehun Han2, Gi Jeong Kim3, Yeon Hee Kim3, Jaewon Choi4,*, MinHo Yang1,2,*
-
Journal of Powder Materials 2024;31(4):293-301.
DOI: https://doi.org/10.4150/jpm.2024.00206
Published online: August 30, 2024
1Department of Hydrogen Energy, Dankook University, Cheonan 31116, Republic of Korea
2Department of Energy Engineering, Dankook University, Cheonan 31116, Republic of Korea
3Department of Chemistry, Dankook University, Cheonan 31116, Republic of Korea
4Department of Chemistry and Research Institute of Molecular Alchemy, Gyeongsang National University, Jinju 52828, Republic of Korea
- *Corresponding Author: Jaewon Choi (J. Choi) and MinHo Yang (M.H. Yang) TEL: +82-55-772-1481 (J. Choi), +82-41-550-3685 (M.H. Yang) FAX: +82-55-772-1489 (J. Choi), +82-41-559-7914 (M.H. Yang) E-mail: cjw0910@gnu.ac.kr (J. Choi), mhyang@dankook.ac.kr (M.H. Yang)
• Received: July 26, 2024 • Accepted: August 19, 2024
© The Korean Powder Metallurgy & Materials Institute
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 11,819 Views
- 288 Download
- 3 Crossref
Figure & Data
References
Citations
Citations to this article as recorded by 

- A facile synthesis of bulk LiPON in solution for solid-state electrolytes
Osma J. Gomez, Adam Antar, Alex T. Hall, Leopoldo Tapia-Aracayo, Joshua Seo, Nam Kim, Zihan Sun, Ryan Lim, Fu Chen, Yue Li, John Cumings, Gary Rubloff, Sang Bok Lee, David Stewart, Yang Wang
Journal of Materials Chemistry A.2025; 13(34): 28368. CrossRef - Uniform lithium deposition using Cu teepee structures for anode-free lithium metal batteries
Seo Yun Jung, Jaehun Han, Seul Ki Choi, Se Youn Cho, Jong Ho Won, Jaewon Choi, Minho Yang
Chemical Engineering Journal.2025; 522: 167302. CrossRef - Garnet-type LLZO electrolytes for solid-state lithium batteries: Interfaces, conductivity, in-situ processing, and industrial prospects
Kaleab Habtamu Ayalew, Nithyadharseni Palaniyandy, Mkhulu K. Mathe, Phumlani F. Msomi
Chemical Engineering Journal.2025; 524: 168098. CrossRef
A Review of Inorganic Solid Electrolytes for All-Solid-State Lithium Batteries: Challenges and Progress
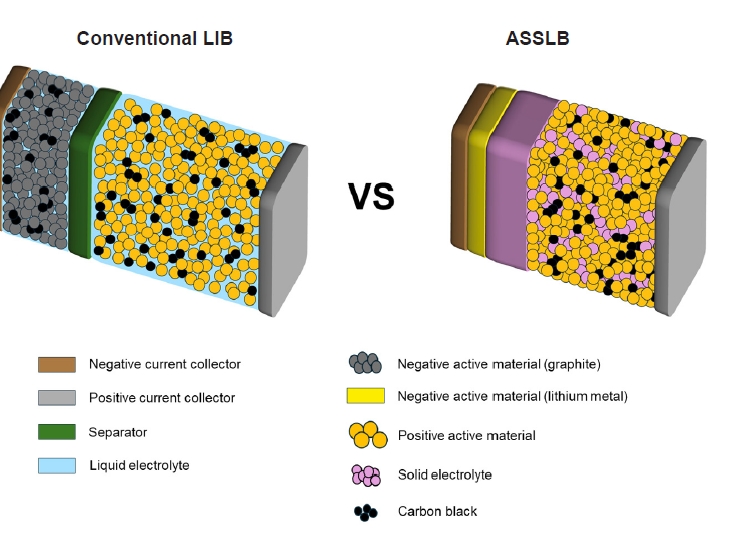
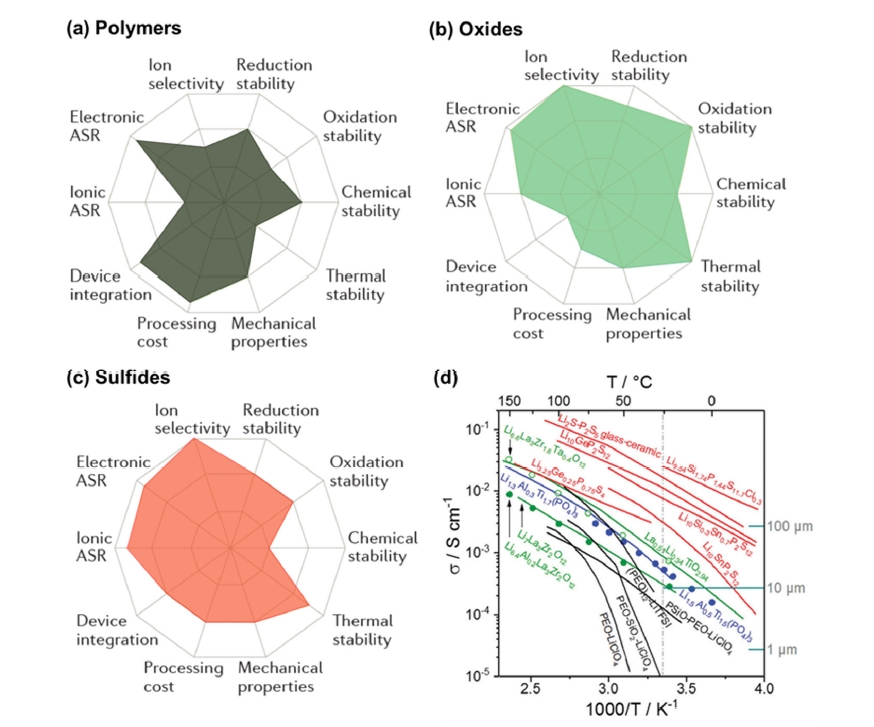
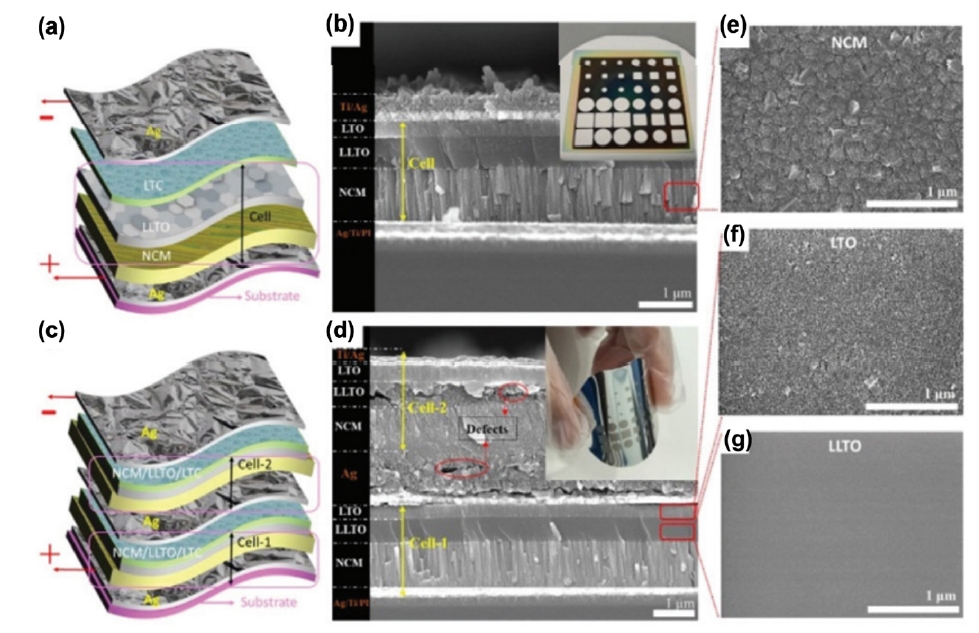
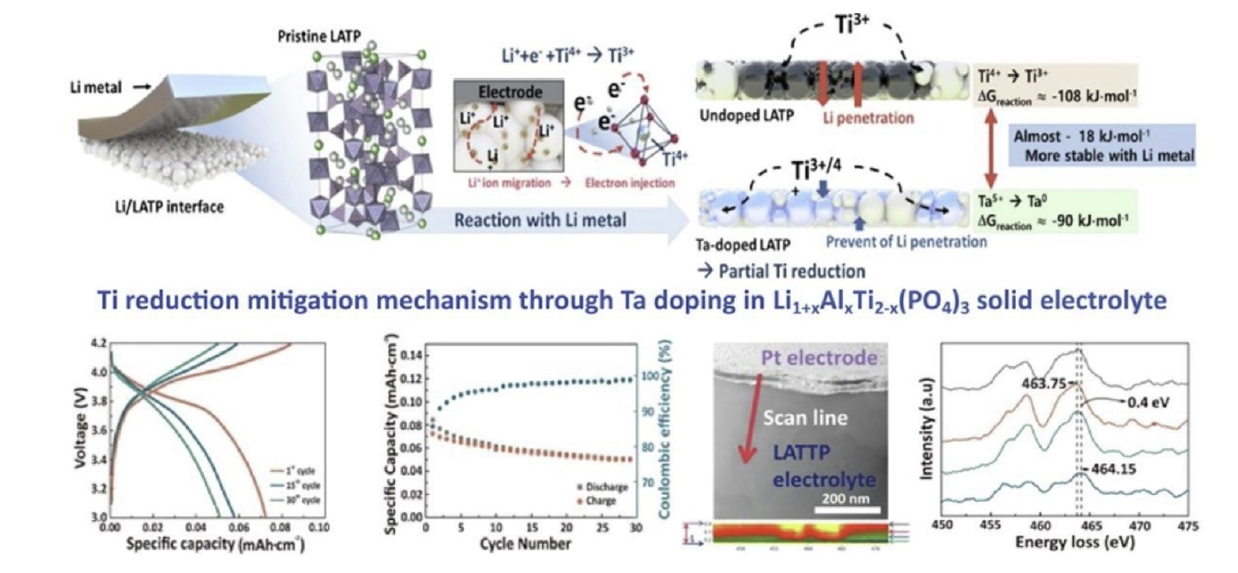
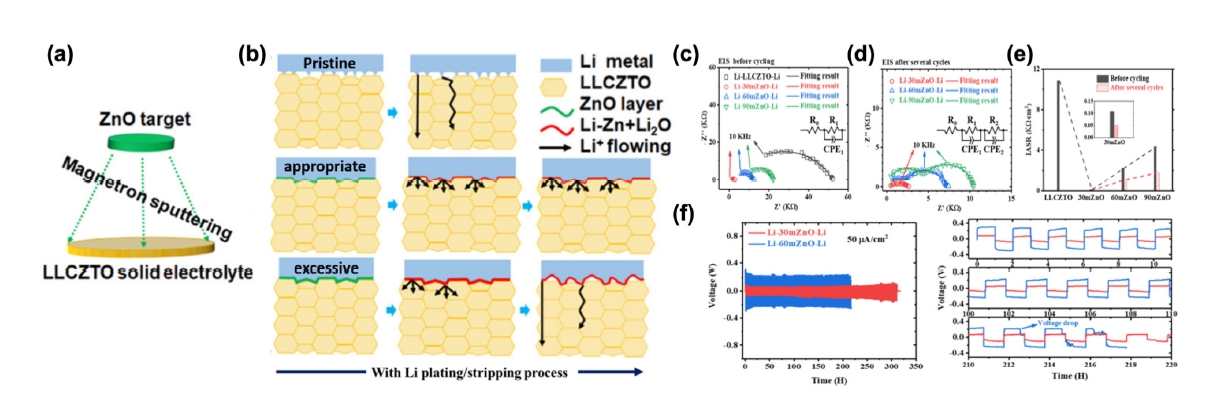
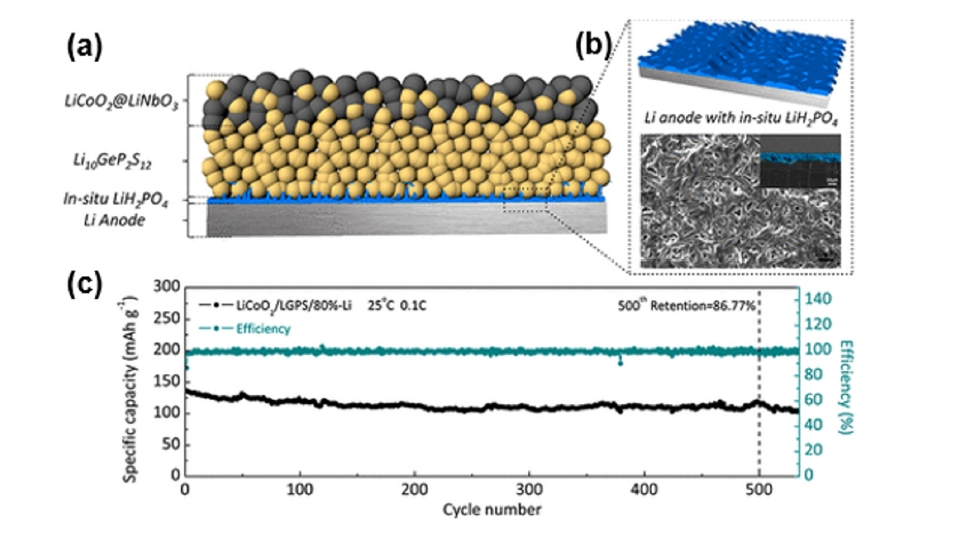
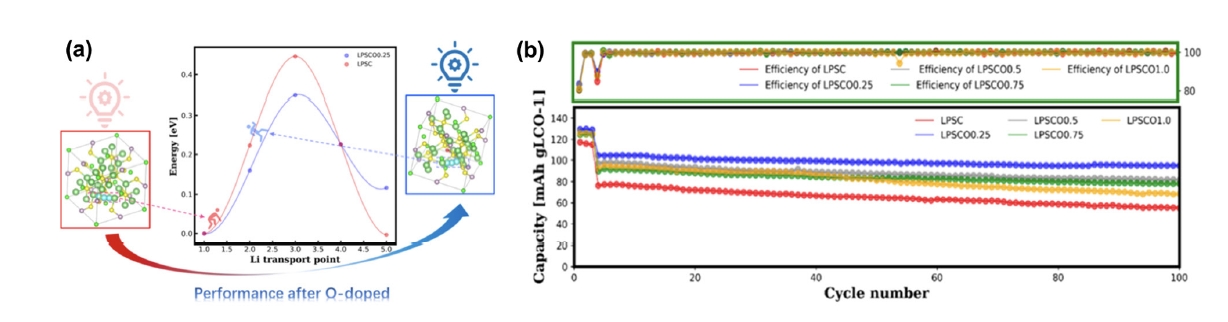
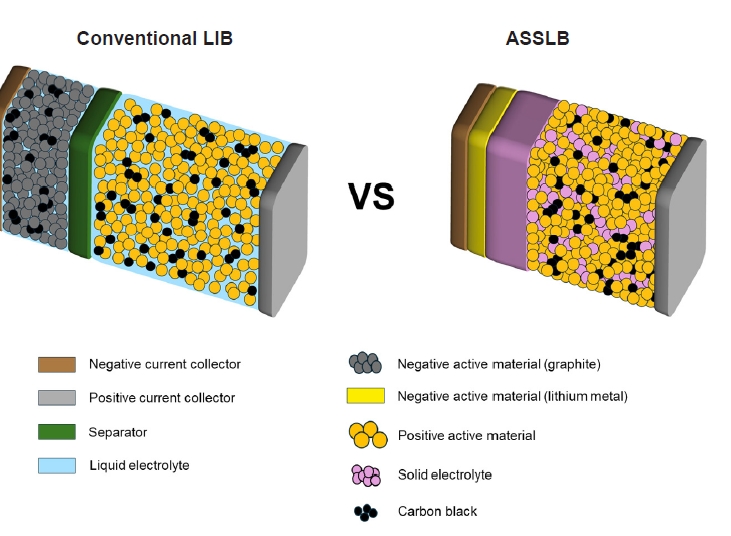
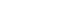
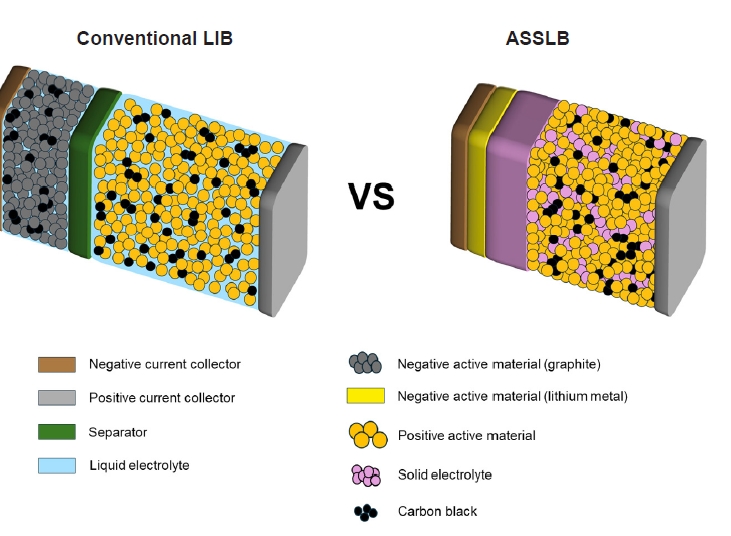
Fig. 1. Schematic diagram of a conventional lithium-ion battery (LIB) and an all-solid-state lithium battery (ASSLB).
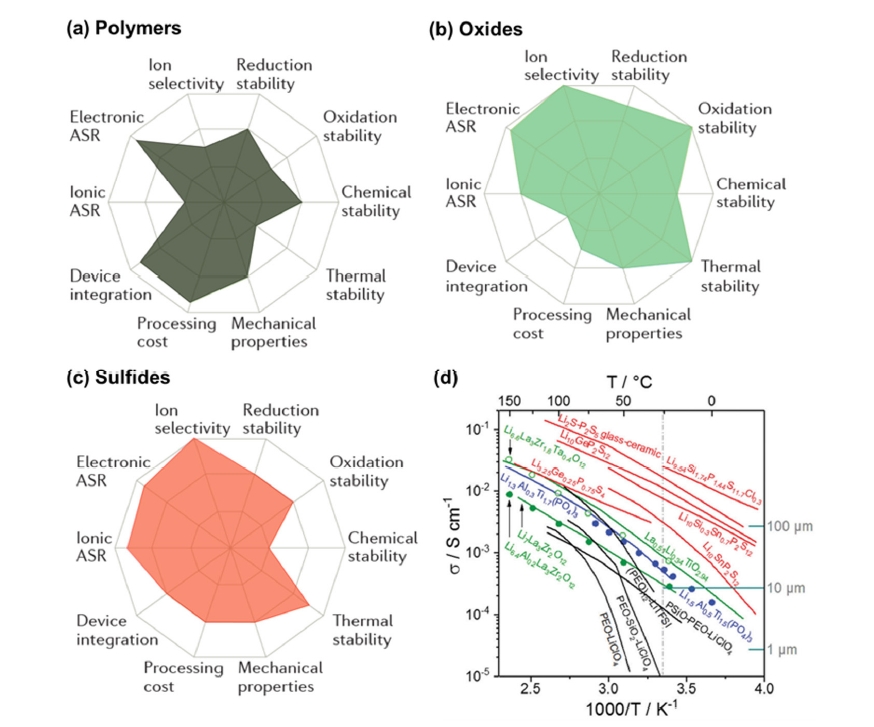
Fig. 2. Comparison of the (a-c) performance [4] Copyright 2017 Springer Nature and (d) conductivity of various solid-state electrolytes (ASR: area-specific resistance, black: polymers, green and blue: oxides, red: sulfides) [5] Copyright 2019 The Royal Society of Chemistry.
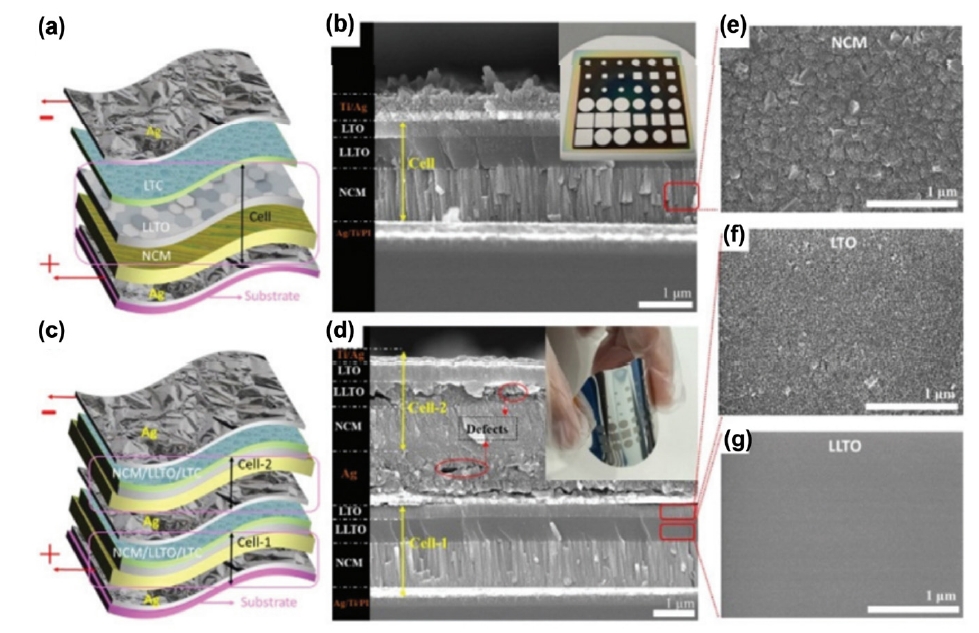
Fig. 3. (a, c) Schematic images of flexible LTO/LLTO/NCM-based all-solid-state lithium batteries (ASSLBs). (b, d) Cross-sectional scanning electron microscopy (SEM) images of LTO/LLTO/NCM-based ASSLBs. (e-g) Top-view SEM images of NCM, LTO, LLTO, respectively [19]. Copyright 2021 The Royal Society of Chemistry.
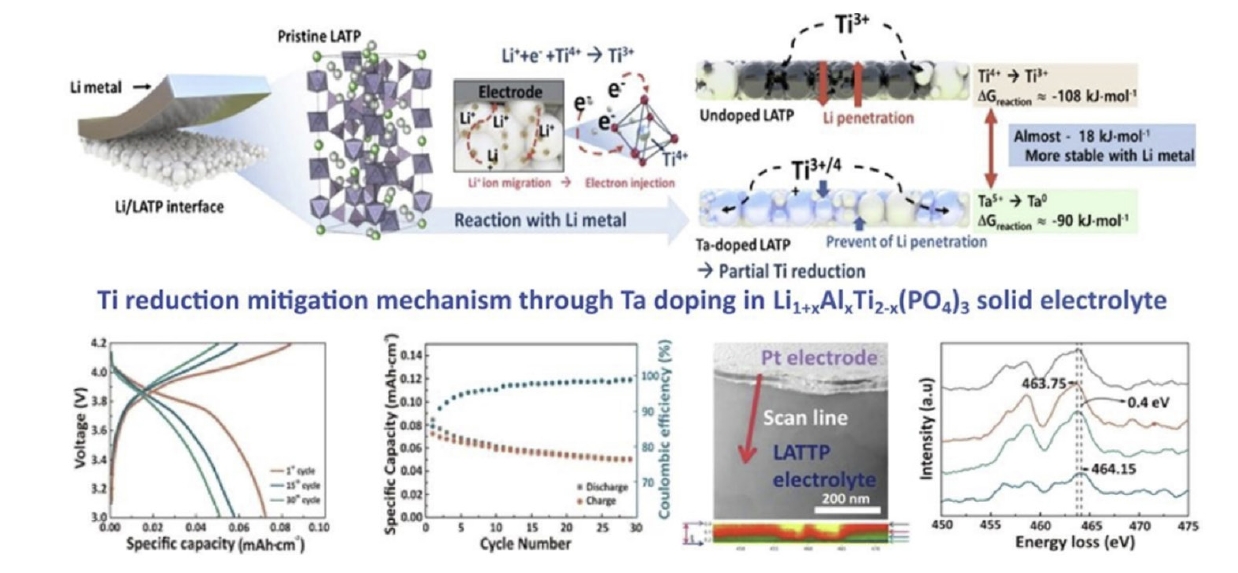
Fig. 4. Schematic images of the Ti reduction–mitigating mechanism though Ta doping in Li1+xAlxTi2-x(PO4)3 solid electrolyte [30]. Copyright 2023 Elsevier.
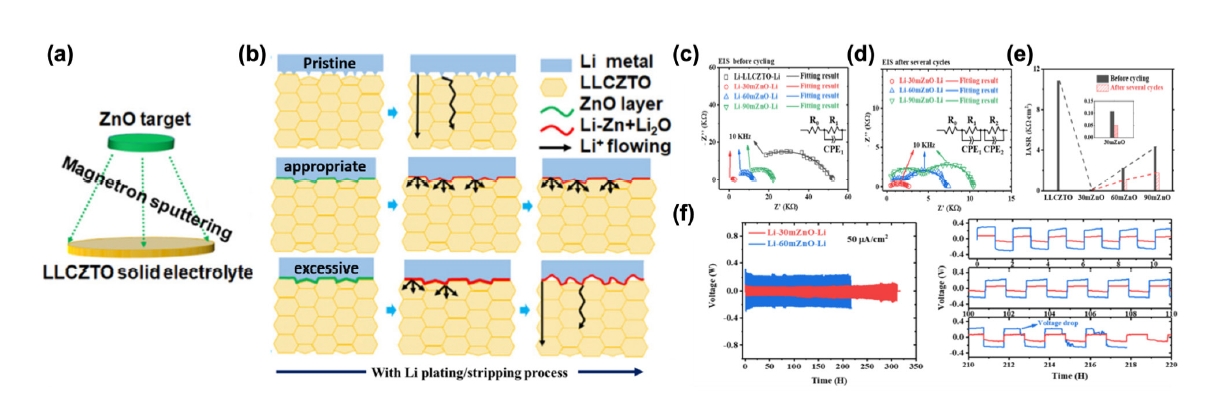
Fig. 5. (a) Schematic illustration of the method of ZnO coating on LLCZTO. (b) Schematic illustration of the evolution of Li/LLCZTO interfacial contact and Li metal growth through LLCZTO with different ZnO thicknesses during the Li plating/stripping process. Nyquist plots of Li−LLCZTO−Li and Li−ZnO−LLCZTO−ZnO−Li cells with different thicknesses (c) before cycling and (d) after several cycles. (e) Change rule of the corresponding interfacial area specific resistance (IASR). (f) Cycling performance of the Li−30mZnO−Li and Li−90mZnO−Li cells at current densities of 50, 100, and 200 μA/cm2 [45]. Copyright 2020 American Chemical Society.
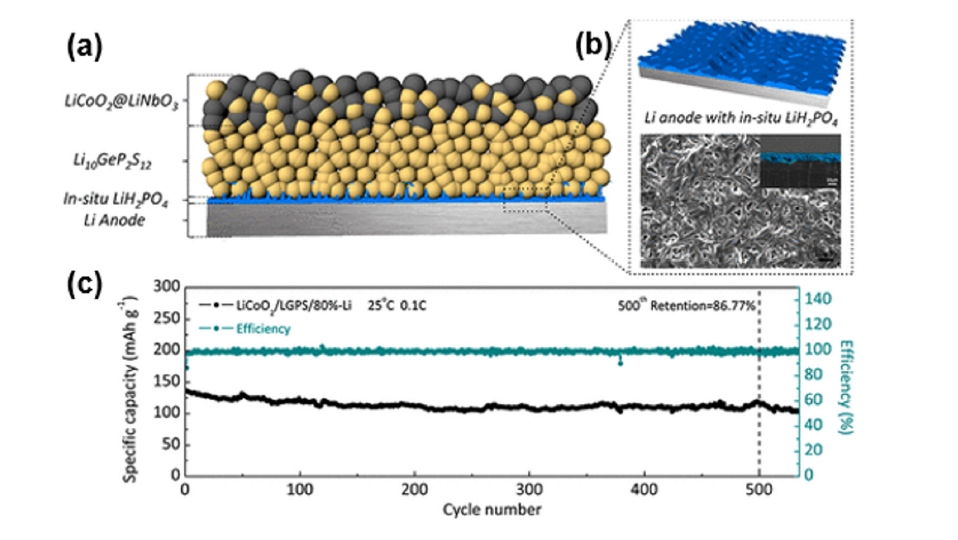
Fig. 6. (a) Schematic of the LCO/LGPS/LiH2PO4–Li all-solid-state lithium battery (ASSLB) with an optimized structure. (b) Preparation process of in situ LiH2PO4 protective layer. (c) Long cycle performance of a LCO/LGPS/80%-Li cell [51]. Copyright 2018 American Chemical Society.
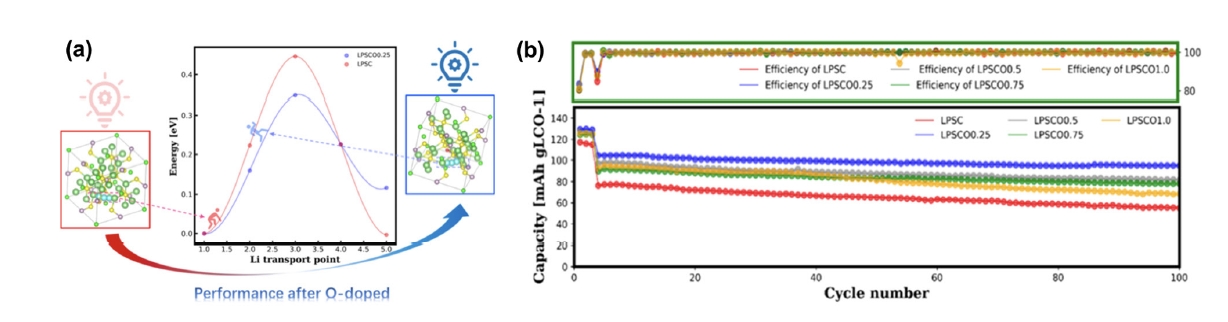
Fig. 7. (a) Li+ migration barrier between the Li6S cages of LPSC and LPSCO0.25 by a density functional theory (DFT) calculation with the climbing image nudged elastic band (CI-NEB) method (blue atoms represent the popping trajectory of Li+). (b) cycling performance of all the LCO/LPSCOx cells for 100 cycles at 0.3 C rate at 25°C [61]. Copyright 2021 American Chemical Society.
Fig. 1.
Fig. 2.
Fig. 3.
Fig. 4.
Fig. 5.
Fig. 6.
Fig. 7.
A Review of Inorganic Solid Electrolytes for All-Solid-State Lithium Batteries: Challenges and Progress
TOP
 KPMI
KPMI

 ePub Link
ePub Link Cite this Article
Cite this Article