Articles
- Page Path
- HOME > J Powder Mater > Volume 32(3); 2025 > Article
-
Research Article
- Self-Assembled Monolayers in Area-Selective Atomic Layer Deposition and Their Challenges
- Si Eun Jung, Ji Woong Shin, Ye Jin Han, Byung Joon Choi*
-
Journal of Powder Materials 2025;32(3):179-190.
DOI: https://doi.org/10.4150/jpm.2025.00094
Published online: June 30, 2025
Seoul National University of Science and Technology, Seoul 01811, Republic of Korea
- *Corresponding author: Byung Joon Choi TEL: +82-2-970-6641 E-mail: bjchoi@seoultech.ac.kr
© The Korean Powder Metallurgy & Materials Institute
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 4,264 Views
- 150 Download
- 1 Crossref
Abstract
- Area-selective atomic layer deposition (AS-ALD) is a bottom-up process that selectively deposits thin films onto specific areas of a wafer surface. The surface reactions of AS-ALD are controlled by blocking the adsorption of precursors using inhibitors such as self-assembled monolayers (SAMs) or small molecule inhibitors. To increase selectivity during the AS-ALD process, the design of both the inhibitor and the precursor is crucial. Both inhibitors and precursors vary in reactivity and size, and surface reactions are blocked through interactions between precursor molecules and surface functional groups. However, challenges in the conventional SAM-based AS-ALD method include thermal instability and potential damage to substrates during the removal of residual SAMs after the process. To address these issues, recent studies have proposed alternative inhibitors and process design strategies.
- Atomic layer deposition (ALD) is a well-established and widely used method to deposit a variety of thin film materials with excellent thickness control, uniformity, and conformality. ALD is extensively used in the production of today’s electronic devices [1-4]. Therefore, ALD can produce highly uniform thin films with a high aspect ratio for a wide range of materials, including oxides, nitrides, sulfides, and pure metals, making it a widely used deposition method in the fabrication of today’s electronic devices [1, 4, 5].
- However, ALD lacks the ability to selectively deposit thin films on target areas. The approach of depositing materials only on desired areas can be achieved through area selective atomic layer deposition (AS-ALD). AS-ALD is a process that selectively deposits a film only on specific substrate areas [1, 2, 5-7]. In the deposition process, the "bottom-up" approach is utilized to reduce additional post-processing steps, such as photolithography and etching, thus significantly shortening the overall manufacturing time. In multilayer patterning, AS-ALD facilitates the self-alignment of 3D structures and offers the potential for the bottom-up fabrication of 3D materials in various nano-fabrication applications [9-13]. Therefore, it can meet the increasing demands of semiconductor production, making it cost-effective [9, 13]. Nanopatterning using AS-ALD is expected to facilitate the fabrication of next-generation electronic and sensing devices, particularly for 2D or 3D metal/dielectric patterns in integrated circuits, transistor backends, interconnects, and FinFET structures. Additionally, it enables the production of higher transistor stacks [13-15].
- AS-ALD includes both surface activation and surface deactivation methods [16]. This paper aims to introduce AS-ALD through surface deactivation. The surface deactivation method selectively blocks nucleation in the non-growth surface (NGS) of the substrate during the ALD process, allowing materials to be selectively deposited only in the growth surface (GS). In this case, deactivation can be achieved by chemically passivating the top surface of the NGS using inhibitors such as self-assembled monolayers (SAMs) [1, 4, 5, 17]. Achieving AS-ALD using SAMs to block ALD typically requires the starting substrates to have significantly different chemical properties [18]. A common reason for the loss of selectivity during deposition is the alteration of surface properties in areas where deposition should not occur upon exposure to ALD chemistry.
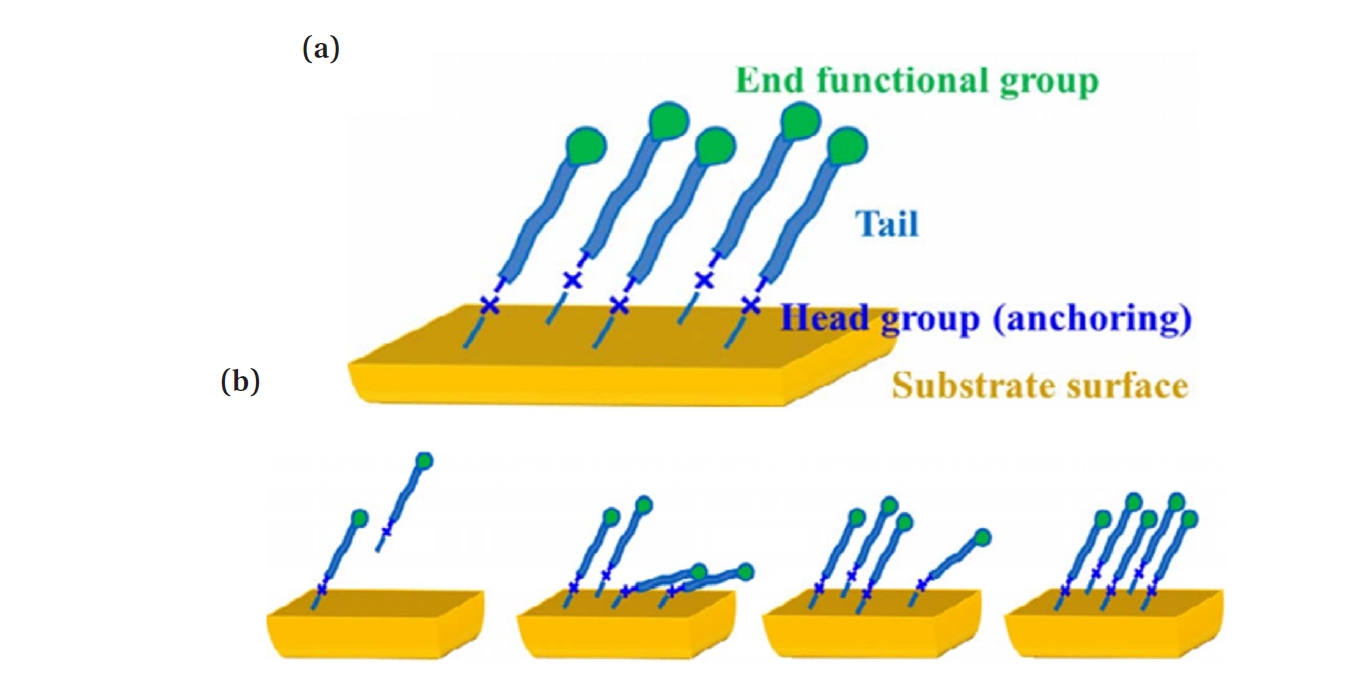
- In recent years, active research has focused on AS-ALD processes utilizing SAMs as chemically selective masking layers to prevent deposition on undesired surfaces [4]. SAMs are used to deactivate the surface to ALD growth [19]. The use of SAMs is strongly related to the chemical and physical stability of thin films. SAM is a spontaneously formed, regularly well-aligned organic molecular thin film on the surface of a given substrate, with the structure of SAM shown in Fig. 1(a) [20]. SAMs are organic films composed of amphiphilic molecules, which consist of three essential components. The reactive headgroup, also referred to as the anchoring group, enables selective binding to the substrate surface. The tail group ensures that the film remains inert to ALD chemistry, while the backbone facilitates the formation of a densely packed monolayer through van der Waals dispersion forces [19]. SAM forms a strong molecular film through direct chemical bonds between the molecules and the substrate surface. Since it is not affected by the shape or size of the substrate, it can be fabricated on substrates with complex shapes and is also suitable for large-area applications [21].
- More commonly, the substrate surface is chemically modified using SAMs [10]. Because of differences in the chemical affinity between SAM molecules and various substrates, selective SAM formation on one surface over another has been demonstrated and used to enable inhibition for AS-ALD [18]. ALD combined with SAMs passivation allows selective deposition on patterned substrates at the nanoscale, enabling bottom-up material fabrication for various applications [3]. SAMs can be removed after the completion of the ALD process through laser irradiation [20], e-beam patterning [17], sonication in ethanol [5], treatment with alcohol or acetic acid etching [28], oxidative methods [29, 30], or by using acetone and deionized water. The appropriate method for removing residual SAMs varies depending on the type of SAM.
- Fig. 1(b) is a schematic of the self-assembly mechanism of SAM. In process i), SAM monomers are applied to the substrate and physically adsorbed. Then, in process ii), chemical adsorption occurs due to covalent bonding between the lying SAM molecules and the substrate, and island-preferred nucleation takes place. In process iii), monomers adsorb around the nucleus and grow in the form of islands, gradually coating the substrate surface. As a result, in process iv), a monolayer with a densely packed, ordered structure is formed [31].
- The previous paper by Jo et al. reviewed AS-ALD for single metal materials [16]. This paper focuses on SAMs and aims to present the conditions for effective AS-ALD using SAMs and precursors, as well as the issues and limitations related to the implementation of the process.
1. Introduction
- This section aims to discuss the functions and requirements of SAM structures for enhancing the selectivity of the AS-ALD process, as well as the conditions of the precursors.
- 2-1. Condition of head group
- The head group of SAMs is the part that chemically adsorbs onto the surface, adhering to all areas of the substrate and ultimately forming a close-packed monolayer [32]. The head group must firmly adhere to the substrate surface for subsequent processes to proceed smoothly and for the formation of a uniform and stable layer. In this case, the head group selectively absorbs onto specific areas of the GS [18]. Table 1 summarizes various studies and presents a compilation of SAM substrate applications based on the head group.
- SAMs with thiol as the head group have been used to deactivate substrates such as Cu, CuO, Cu₂O, and Au. SAMs with phosphonic acid as the head group have been applied to deactivate substrates such as Al₂O₃, Cu, CuO, Co₃O₄, WO₃, and RuO₂. SAMs with alkyl silane as the head group have been used to deactivate substrates like TiN and SiO₂. Finally, SAMs with carboxyl as the head group have been used to deactivate substrates such as Cu and Co. SiO₂ is primarily used as the GS, and the deposition temperature of the precursors in these studies typically ranges from 100 to 200°C [18].
- 2-2. Condition of tail group
- The blocking efficiency of ALD precursors is determined by the packing density of the SAM, with the tail group of the SAM playing a key role in this function. Longer molecular chains of SAMs result in higher packing densities, while variations in chain length can influence their wetting performance [33, 34]. To enhance the selectivity of the process, the steric shape of the tail group plays a critical role, as highlighted by Bent et al [33].
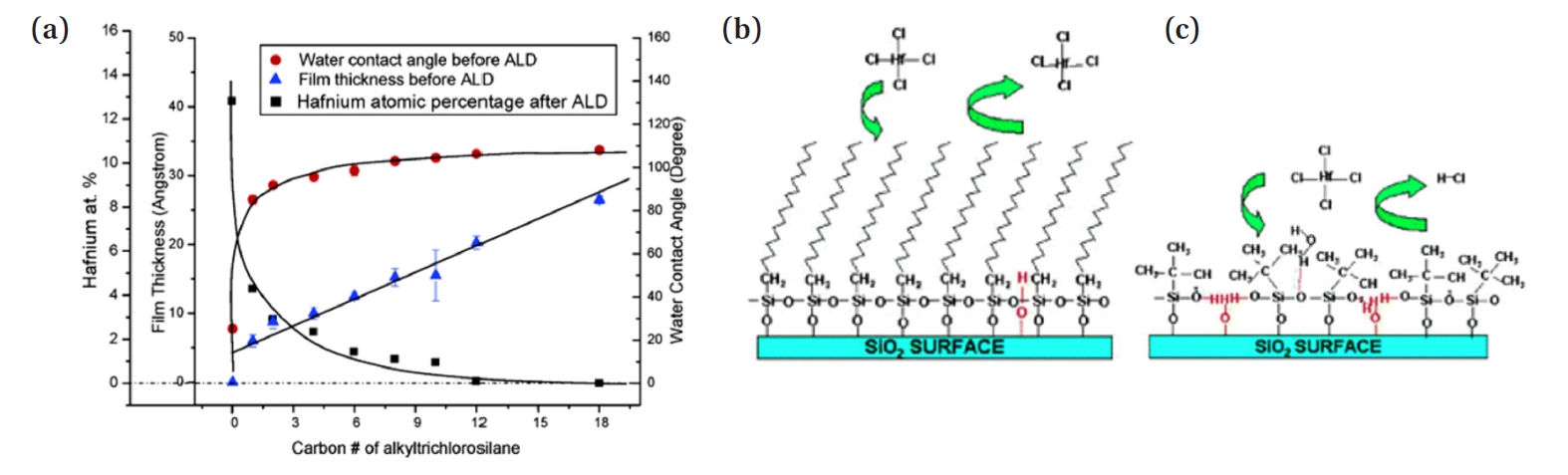
- The packing density of SAMs depends on intermolecular van der Waals (VDW) dispersion forces, which are proportional to the strength of the induced dipole. As shown in Fig. 2(a), the thickness of the SAM film increases linearly with the number of carbon atoms in the Alkylchlorosilane. Conversely, the water contact angle sharply increases upon the addition of the first one or two methyl units to the alkyl chain, followed by a gradual rise until it stabilizes at a plateau, with a static water contact angle (WCA) of approximately 110°. Furthermore, the Hf atom % of the ALD precursor in the experiment decreases inversely with the WCA, indicating that as the length of the tail group of the deposited SAM increases, the precursor's blocking efficiency improves. The strengthening of VDW interactions with increasing alkyl chain length explains this phenomenon. Longer alkyl chains exhibit stronger VDW interactions due to the greater number of electrons and the extended molecular structure, which allows for more extensive induced dipole interactions. In contrast, shorter-chain deactivating agents experience relatively weak interchain VDW attraction, as evidenced by their lower packing density and reduced hydrophobicity, as indicated by WCA measurements. As a result, for molecules with the same tail group structures, longer alkyl chains enhance deactivation efficiency.
- Moreover, since molecular structure also affects the strength of intermolecular VDW interactions, the shape of the tail group in SAMs is a key factor in selecting effective deactivating agents. In the case of Fig. 2(b), using a long linear chain as the tail group effectively blocks the precursor from penetrating the monolayer. On the other hand, in Fig. 2(c), the large volume of the tail group leads to a lower packing density, resulting in surface defects and pinholes. As a result, the precursor penetrates the SAM, binds with the substrate surface, and decreases the selectivity of the process [33]. To use SAM as an ALD monolayer resist, a thin film with high packing density is required. Therefore, to obtain the most closely packed film, simple, linear alkyl chain groups are necessary.
- Therefore, the longer the chain length and the simpler the linear shape of the SAM's tail group, the higher the packing density and the better the blocking efficiency. For this reason, linear alkyl chain groups like C18 (octadecyl group) have been widely used in many studies.
- 2-3. Condition of functional group
- To enhance the deactivation effect through SAMs, the design of the functional group, also known as the end group, is crucial. Selective deposition can be realized by manipulating, prior to deposition, surface functional groups according to a chosen pattern to either block or allow film growth as desired [5]. This functional group is located on the opposite side of the part of the SAM that interacts with the substrate, and in AS-ALD, it plays a role in controlling the reactivity with the ALD precursor. In other words, due to the well-ordered structural characteristics of the SAM, when the terminal functional groups are exposed to the surface, the surface properties can be controlled at the molecular level using these groups [32]. Inhibiting surface reactions with the precursor to enhance deactivation efficiency leads to improved selectivity. The conditions for the functional groups to increase deactivation efficiency are as follows.
- Firstly, a SAM should be used that has no thermodynamic driving force for the reaction and a large reaction barrier. Table 2 presents the reactivity of OTS-based SAMs with different terminal groups (-OH, -NH2, -CH3) with TMA precursors [14]. A lower proton affinity indicates that less energy is required for the TMA reaction. The barrier energy shown in the table is the energy barrier for the reaction between TMA and the terminal group, and it is directly proportional to the proton affinity. The total energy at the end reflects the calculated trend for activation energy. A negative value indicates that the state where TMA and the terminal group are bound is stable, while a positive value suggests that the dissociated state is more stable.
- OH-terminated SAM has a very low energy barrier, so when heat is applied, it reacts easily with TMA and becomes adsorbed. The reaction path of NH2-terminated SAM with TMA is similar to that of OH-terminated SAM with TMA; however, for -NH2, the reaction barrier energy is higher, necessitating elevated temperatures for the reaction to occur. CH3-terminated SAM has not only a very high reaction barrier energy but also no thermodynamic driving force for adsorption. In conclusion, within the comparison groups of this study, CH3-terminated SAM can be considered the most efficient SAM. Therefore, to enhance deactivation efficiency through SAMs, it is important to design the terminal groups by considering the reaction kinetics with the precursor.
- Additionally, this study found that the rate constant for the reaction between the terminal groups and TMA varied with temperature. Subsequently, TMA can be selectively adsorbed onto the SAM or the exposed substrate, depending on the functional group's affinity for TMA. Finally, ALD can be performed on the adsorbed state to grow uniform, thin Al2O3 films.
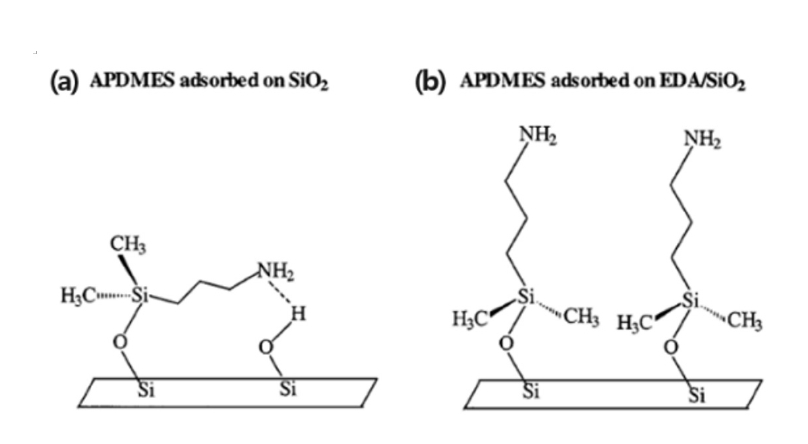
- The second condition is that for SAM to be completely well-ordered, the compatibility reaction between the chemical functional group of the substrate surface and the terminal group of the SAM is crucial. Fig. 3 describes the deposition of an APDMES SAM with an NH2 terminal group on a silica surface [35]. Fig. 3(a) shows a schematic in which the amino-terminal group forms a hydrogen bond with the silanol groups on the SiO₂ surface, preventing the formation of a well-ordered SAM. This suggests that, to achieve a well-ordered SAM, it is important to design terminal groups that are unreactive with the substrate.
- However, Fig. 3(b) shows an example where a well-ordered monolayer is formed even with a terminal group that is reactive with the substrate. Before applying the SAM, a catalytic substance, ethylenediamine (EDA), is first attached to the surface. This EDA modifies the silanol group at the Si surface to an amino-propyl group, preventing the amino-terminal group of APDMES from adsorbing onto the substrate. Additionally, the monoethoxy head group catalyzes reactions, facilitating the formation of a well-ordered SAM. Therefore, to achieve a well-ordered SAM, it is crucial to design an end group that is non-reactive with the substrate. However, if a reactive terminal group is employed, surface treatment of the substrate can enable the formation of a well-ordered SAM [35].
- 2-4. Condition of ALD precursor in AS-ALD
- The conditions of the ALD precursor during AS-ALD are outlined below. If the precursor molecule is small, it can more easily penetrate the SAM during the deposition process, potentially affecting passivation. Additionally, both ALD and AS-ALD processes are highly dependent on ALD chemistry. For instance, nucleation and growth behavior vary depending on the precursor type and process temperature during material deposition.
- The study shown in Table 3 discusses the influence of precursor design on AS-ALD [36]. Al₂O₃ was deposited on a SiO₂/Si substrate using five types of SAMs: Al(CH3)xCl3-x (x = 0, 2, and 3) and Al(CyH2y+1)3 (y = 1 and 2). The selectivity was evaluated using an ODTS SAM. The five precursors used in this experiment are all aluminum-based but contain different ligands. As a result, their reactivity and molecular size vary. This study aims to examine how the reactivity and molecular size of aluminum precursors influence the SAM during AS-ALD.
- First, the role of Lewis acidity in the physical adsorption of chloride precursors on SAM-passivated surfaces is investigated. The Lewis acidity of aluminum precursors varies for each precursor. Due to the high electronegativity of the Cl ligand, the Lewis acidity of Al(CH₃)ₓCl₃₋ₓ decreases as the number of chlorine ligands decreases. Consequently, an increase in chloride ligands leads to greater precursor adsorption on the surface, reducing the effectiveness of precursor blocking. Among the aluminum precursors examined in this study, AlCl₃, which exhibits the highest Lewis acidity, may lead to unintended Al₂O₃ nucleation if not sufficiently purged.
- Additionally, the strong Lewis acidity of chlorine ligands facilitates precursor dimerization, resulting in a larger effective volume (Veff). The increase in Veff of such chlorine-based precursors enhances the blocking effect; however, due to their strong Lewis acidity, they adsorb more readily on the surface and require longer purge times. Therefore, precursors with chlorine ligands cannot be considered highly effective.
- Next, the study aims to investigate how precursor size and dimerization, in addition to Lewis acidity, influence precursor blocking during AS-ALD. To understand the thermodynamic equilibrium of dissociation, the Gibbs free energy for the dissociation of dimers to monomers of the five precursors was calculated at 200°C (Table 3). According to the calculation results, the dissociation ΔG of Al (CH3)3 and Al(C2H5)3 precursors is negative. Therefore, the dissociation reaction to a monomeric structure occurs spontaneously, and these two precursors predominantly remain in their monomeric form. In contrast, for the other three precursors, the dissociation ΔG for the decomposition of Al dimers is positive, indicating that the dissociation reaction to monomer is not spontaneous. As a result, they predominantly remain in their dimeric form. At the process temperature of 200°C, 99% of the dimers of Al(CH3)3 SAM and Al(C2H5)3 SAM dissociate into monomers, while only 1% of the dimers of the remaining three SAMs dissociate into monomers.
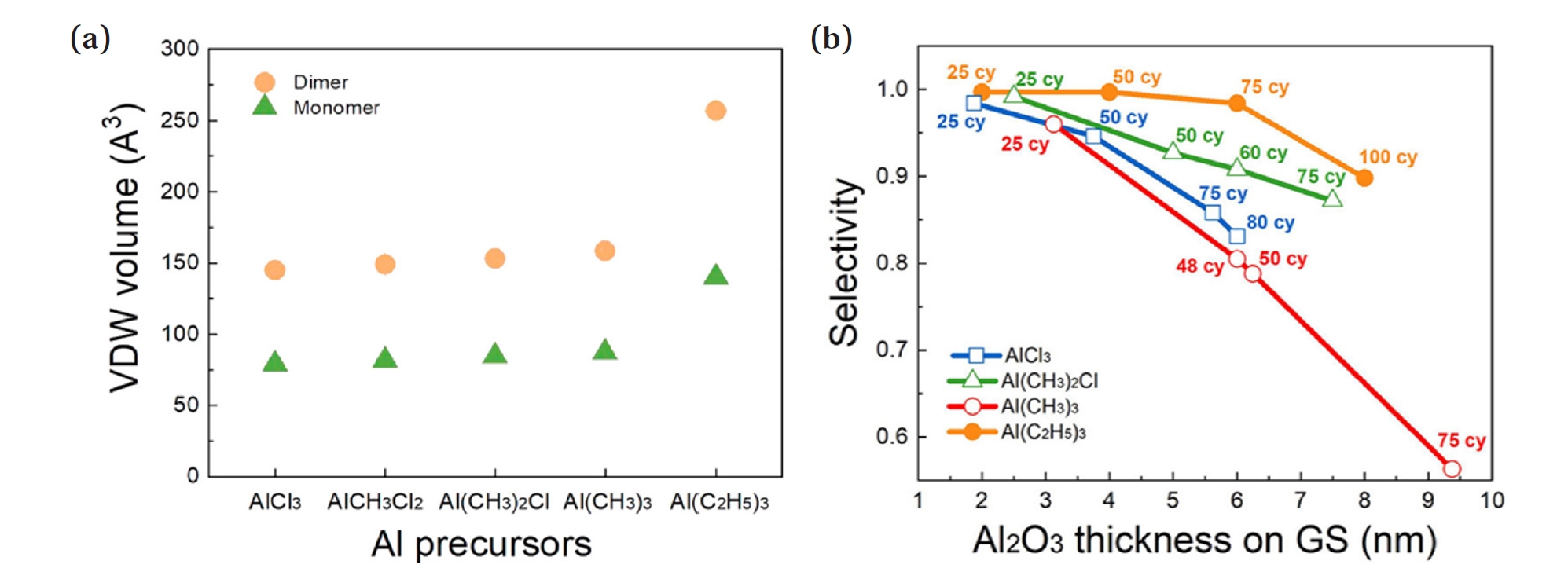
- As shown in Fig. 4(a), the VDW volume of the monomer and dimer for the five aluminum precursors is presented. In this case, for all precursors except Al(C2H5)3, the sizes of the dimer and monomer are similar. Al(C2H5)3, while predominantly remaining in the monomeric form, has a relatively large monomer size. This large volume effect prevents the precursor from penetrating effectively during the ALD process, thereby enhancing selectivity.
- Through this equation (1), considering the relative sizes of Cmonomer, Cdimer, and the precursor, the effective average size of the precursor molecule, Veff, can be calculated. This Veff value represents the average size of the precursor molecule that can exist at 200°C and serves as an indicator of how efficiently the AS-ALD process can be conducted through the SAM. When the precursor size is small at the ALD process temperature, the precursor can easily penetrate the SAM during the process, potentially affecting passivation. Therefore, precursors that maintain a larger volume during the AS-ALD process lead to higher selectivity.
- In Table 3, the effective volumes of Al(CH₃)₃ and Al(C2H5)3 are 87.2 and 140.2, respectively. These represent the precursors with the smallest and largest molecular sizes. By comparing selectivity between these two precursors, the blocking effect related to precursor size is examined. The experimental results show that, even with a higher number of cycles and thicker alumina layers, Al(C2H5)3 exhibits the highest selectivity, while Al(CH3)3 shows the lowest selectivity (Fig. 4(b)). This indicates that when a larger molecular size SAM is deposited, better selectivity is achieved. Additionally, during the ALD process, longer purge time and higher pressure of the Ar gas used during purging allow for more effective blocking of the precursor. Therefore, Al(C2H5)3, with a larger molecular size compared to Al(CH₃)₃, more effectively prevents diffusion into the SAM.
- Il-Kwon Oh et al. explains the influence of precursor design, including reactivity and molecular size, on the blocking effect during AS-ALD [36]. While the size of the precursor molecule is the most dominant factor influencing selectivity in terms of adsorption by the SAM, the reactivity of the precursor, such as its Lewis acidity, also plays a crucial role in determining the blocking ability. Among the five aluminum precursors, Al(C2H5)3, which has a low Lewis acidity and relatively large molecular size, exhibited the best blocking effect. In other words, the blocking effect is improved when the precursor has low Lewis acidity, large effective volume, high purge pressure, and long purge time during the process. Therefore, when performing the AS-ALD process using SAM, the design of the precursor, including its reactivity and molecular size, must be carefully considered.
- ALD precursor choice can also negatively affect SAM stability depending on the SAM molecule being used. For example, ALD precursors containing chloride ligands may reduce the stability of SAMs adsorbed on metal oxides. This instability arises from the generation of HCl gas as a primary by-product when water is used as a coreactant with chloride-based precursors, potentially leading to surface etching. To ensure the stability of SAMs under ALD conditions, it is crucial that they form strong, high-density surface bonds with the underlying substrate, thereby minimizing the formation of surface defects. Extensive research on SAMs as inhibitors for AS-ALD has contributed to identifying two important factors in the interaction between precursors and inhibitors that enhance selectivity.
- First, preventing the formation of access points that could lead to defects within the inhibitor layer is essential for maintaining its blocking performance. Second, this defect prevention is most easily achieved by maximizing the areal density of inert blocking groups at the interface between the inhibitor and the precursor [17, 37]. Finally, the precursor should be designed such that its effective volume is larger than the gap between the inhibitors at the temperature where ALD is performed. Kim et al. investigated ALD using TMA and dimethylaluminum isopropoxide (DMAI) precursors on substrates inhibited by ethanethiol (ET) [38]. They found that ET was much more effective at inhibiting deposition when using DMAI compared to TMA. This is because, at the ALD temperature, most of DMAI exist as dimers and is easily blocked by ET, while the majority of TMA exists as monomers, which can enter the gaps between the ET molecules, adsorb, and initiate nucleation.
2. Surface deactivation using SAM
- Several issues must be addressed to implement the deactivation AS-ALD process using SAM. This section discusses various process challenges encountered in AS-ALD through SAM and the research aimed at improving these challenges.
- 3-1. Thermal Instability of SAM
- Thermal stability is desirable for future molecular electronics applications [15]. The low thermal stability of SAMs severely limits their applicability for achieving area-selective ALD, as most ALD processes have temperature windows in the range of 100−400 °C [12]. As each SAM has a different stable temperature, its passivation properties degrade, leading to precursor penetration and adsorption, which can cause film growth even in NGS.
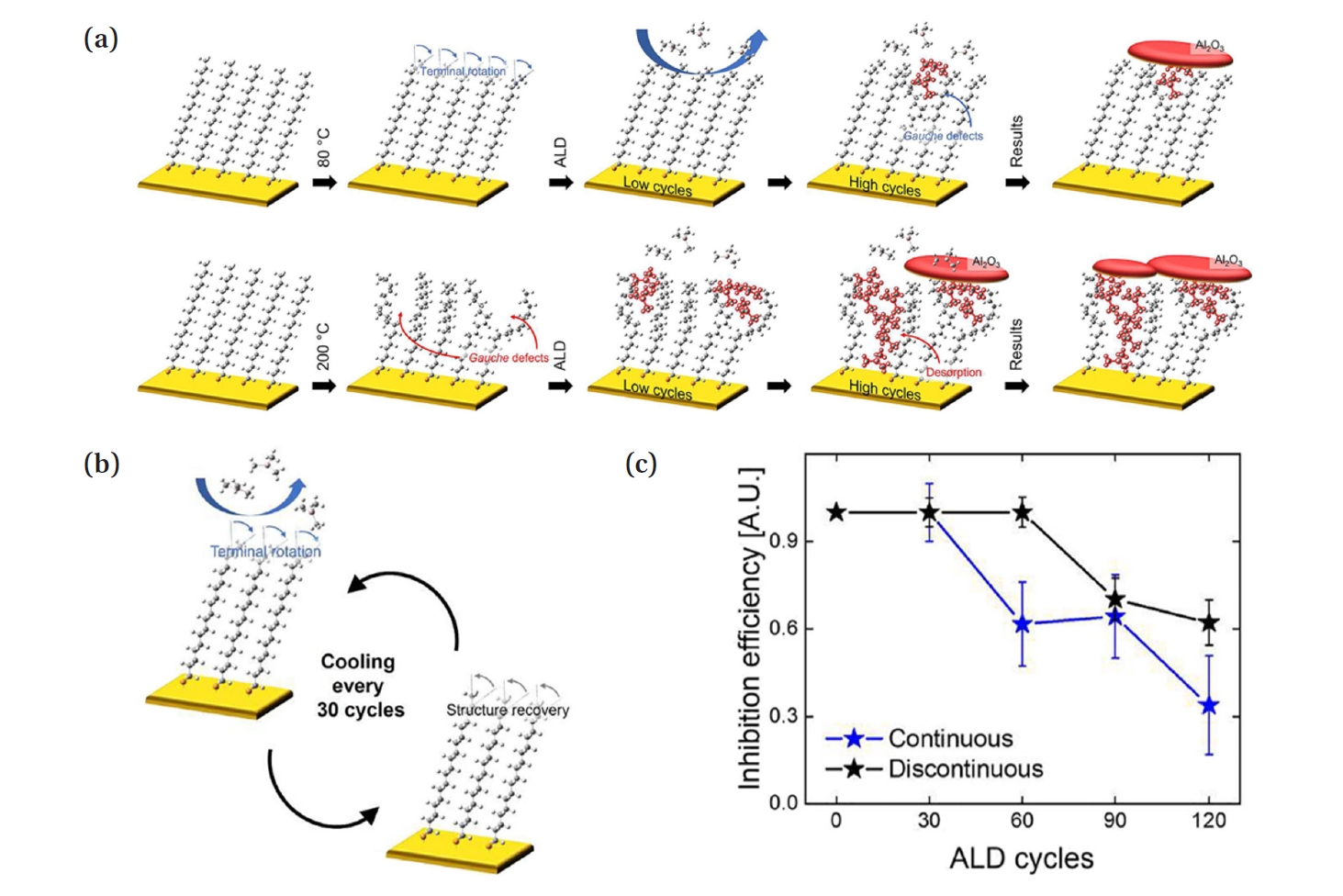
- Choi et al. reveals the mechanism of structural changes in SAMs caused by heat [13]. Fig. 5(a) illustrates a schematic showing the loss of passivation functionality in ODT SAMs on an Au substrate during AS-ALD at 80°C and 200°C. When ALD is performed on the ODT SAM at 80°C, the terminal groups (C16-C17 and C17-C18) of the SAM, which have relatively weak VDW forces, begin to undergo internal rotation due to heat. At this point, since the terminal methyl groups undergo reversible rotation, cooling back to room temperature, as shown in Fig. 5(b), allows for the recovery of the structure. However, after about 50 ALD cycles, stress and heat cause the methyl groups to undergo irreversible rotation, causing gauche defects. While the process heat alone does not provide enough energy to overcome the energy barrier for irreversible rotation of the tail group, the additional stress from ALD exceeds the threshold, resulting in gauche defects. When ALD is performed at 200°C on the ODT SAM substrate, gauche defects due to irreversible rotation occur after just five cycles, and as the process continues, not only does the rotation of the tail group increase, but the SAM monomer can also decompose and detach.
- Choi et al. proposes the discontinuous ALD process as a solution, in which the mechanism of SAM passivation loss due to thermal effects is utilized to restore the molecular structure of SAM by periodically cooling the substrate after a certain number of ALD cycles [13]. The molecular structure of the ODT SAM on an Au substrate undergoes reversible internal rotation, which is recoverable by thermal effects, at temperatures below 80°C for up to 50 ALD cycles. In this experiment, the substrate was cooled to room temperature every 30 ALD cycle to restore the SAM structure before resuming the ALD process. As shown in Fig. 5(c), the inhibition efficiency of the SAM in typical ALD decreases to a low value after 60 cycles, while in the discontinuous ALD process, the inhibition efficiency remains close to 1 up to 60 cycles. Here, they showed that the discontinuous ALD scheme is a simple and easy way to increase the inhibition efficiency. However, this method is limited to materials that can undergo ALD at temperatures below 80°C [13].
- Another effective solution to address the thermal instability of SAMs is the correction steps. According to Mackus et al., when the loss of function in the ALD NG region due to heat is inevitable, it can be restored through correction steps [12]. The Correction Steps are divided into two methods. This leads to the development of advanced ALD cycles by combining conventional two-step ALD cycles with correction steps in multistep cycle and/or supercycle recipes.
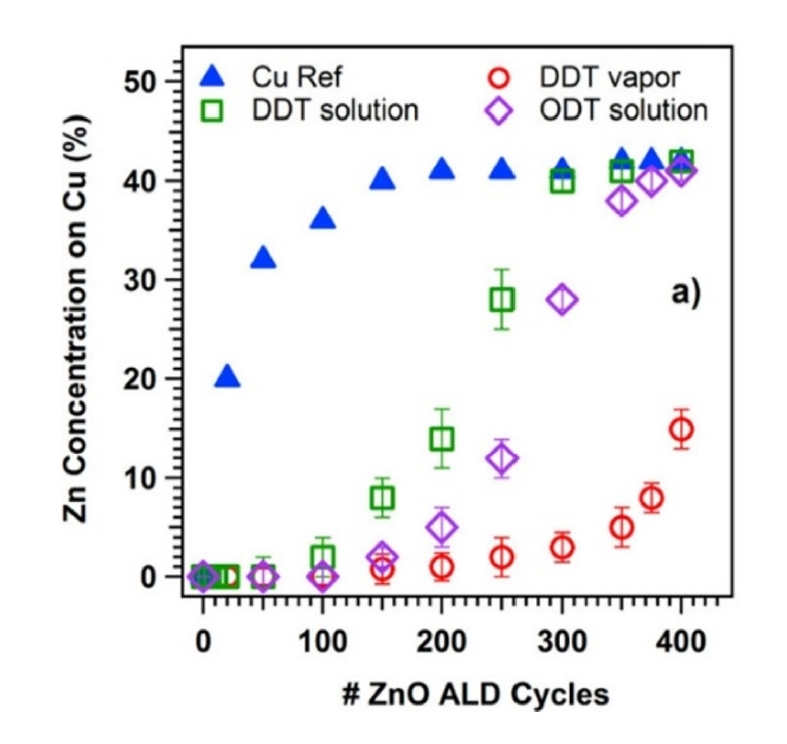
- The first method is repetitive functionalization. In this method, when the SAM monomer detaches to some extent due to heat during the ALD process, SAM is replenished every certain number of cycles to restore the detached SAM. This approach helps maintain the deactivation functionality even at high ALD cycle counts. Hashemi et al. conducted AS-ALD of ZnO on a Si substrate covered with Cu/SiO₂ precursors on substrates inhibited by using repetitive functionalization using octadecylthiol (ODT) and dodecylthiol (DDT) SAMs [1]. The ZnO ALD was deposited for more than 600 cycles, with the DDT SAM undergoing a re-dosing process every 150 cycles, for a total of 4 times, while the ODT SAM underwent a re-dosing strategy every 100 cycles, for a total of 6 times.
- According to the XPS analysis results shown in Fig. 6, the general DDT and ODT SAMs, without re-dosing, lose their blocking effect after approximately 200 and 350 cycles, respectively [1]. However, the DDT and ODT SAMs that underwent re-dosing processes can suppress the growth of ZnO on Cu up to 600 cycles [1].
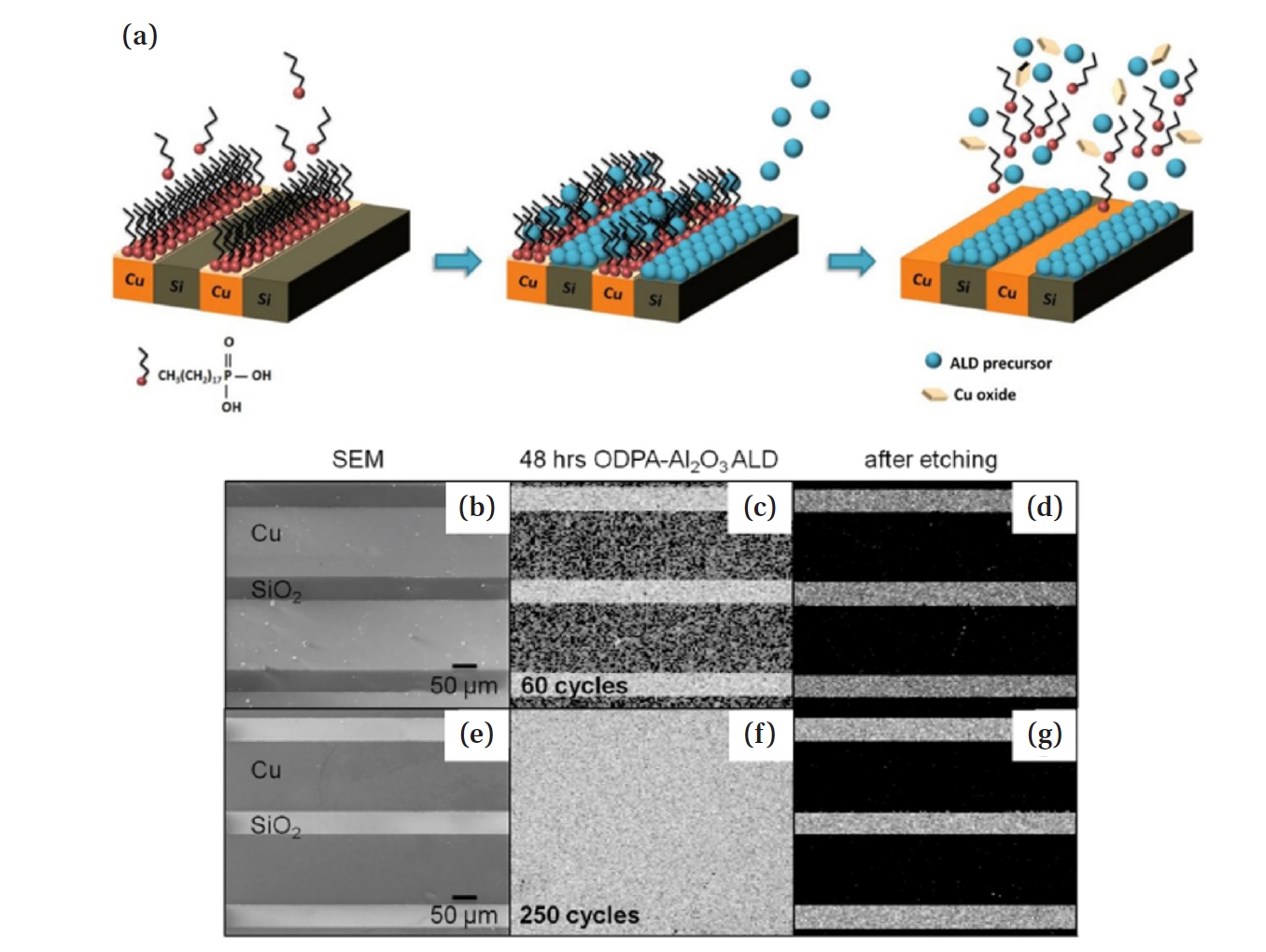
- The second method is selective etching. This approach is applicable to metal/dielectric patterning substrates. It is a method in which the SAM regions damaged by process heat and stress are removed through selective etching during the process, followed by re-patterning the SAM to enable continuous AS-ALD. The process is inherently self-correcting, because undesired deposition can be cleanly removed in the second step, allowing for high quality patterning. Hashemi et al. proposed a method in which, as shown in Fig. 7(a), when the patterned SAM on Cu over Si substrate is damaged through repeated process cycles, a mild etchant such as acetic acid is used to selectively etch only the copper oxide layer, thereby restoring selectivity [28]. They propose and test a combined selective deposition-selective etch approach and show that it improves the film thickness for which selective deposition can be performed at least 10 times. This is a lift-off method in which the natural oxide of Cu is removed, along with the SAM and precursor, simultaneously. The lift-off process exposes the metal surface, and thereafter, the SAM is re-adsorbed, enabling high-selectivity AS-ALD to proceed.
- As shown in Fig. 7(b, e), when ALD was performed on a Cu/SiO2 substrate for 60 and 250 cycles, selectivity was lost as seen in Fig.s 7(c) and (f). However, in both cases, when acetic acid was added, Fig. 7(d, g) demonstrates that only the Cu region was selectively etched [39]. This suggests that even if selectivity significantly decreases due to SAM damage, selectively etching the damaged part during the process can restore the well-ordered SAM structure, allowing for high-cycle AS-ALD with maintained high selectivity.
- Moreover, the required formation time of the SAM resist can be significantly reduced from 48 h to at most 1 h, making the process much more efficient [28]. The selectivity between GS and NGS ultimately diminishes for all known combinations due to defects, the formation of new defects, and the Boltzmann distribution of molecular reactivities on the surfaces. This selectivity can be regained by implementing etch-back correction steps [40, 41].
- 3-2. Residual SAM removal issues
- The elimination of residual monolayers typically requires strong treatments such as plasma [42], UV light [43, 44], ozone [21], or acid etching [28, 30], which can cause substrate damage and present issues in various applications [27-28, 40]. López et al. proposed the use of stearic acid (SA) SAM with a carboxyl group as a headgroup, which can be removed using an appropriate polar solvent, as a solution to this issue, rather than employing surface treatment methods such as UV light or acids [27].
- The study concludes that SA SAM serves as an effective and removable copper passivation agent for AS-ALD without requiring exposure to harsh conditions such as etching or acid treatments [27]. Its chemical structure exhibits the typical characteristics of SAM molecules utilized in AS-ALD, featuring a saturated 18-carbon chain terminating in a carboxylic acid group (CH₃(CH2)16COOH). ODPA and ODT SAMs, which have been frequently studied in AS-ALD, strongly adsorb and are challenging to remove even when immersed in polar solvents such as water, ethanol, or acetone, requiring immersion times of over 1 hour for ODPA and 25 hours for ODT. In contrast, stearic acid SAM can be efficiently removed with a much shorter immersion time of just 25 minutes. This makes stearic acid a promising candidate for AS-ALD, especially when efficient removal of residual SAM is required. Through the experiments conducted, SA SAMs were successfully removed after 40 minutes in deionized water and 30 minutes in ethanol. These results demonstrate that no strong etching treatment is necessary for monolayer removal, offering a new approach for AS-ALD that involves mild monolayer removal.
3. Issues and Improvements in the Implementation of SAM
- This paper examines the key factors for achieving high efficiency and selectivity in deactivation-based AS-ALD using SAMs as inhibitors, with a focus on SAM structure and precursor design. The blocking efficiency of SAMs improves when the tail group is a simple linear chain, while the functional group should neither be thermodynamically reactive with the substrate nor strongly interact with the precursor. Furthermore, the precursor’s reactivity with SAMs and its molecular size within the ALD environment significantly influence selectivity.
- Despite their promising precursor-blocking performance, SAM-based deactivation in AS-ALD faces several implementation challenges. SAMs can develop gauche defects or detach due to ALD heat, necessitating correction steps. Additionally, the removal of SAMs after deposition often requires strong treatments that may damage the substrate; however, SA SAMs, which can be dissolved in polar solvents, present a potential solution.
- A promising alternative is small molecule inhibitors (SMIs), which consist of a chemisorptive reactive moiety and an inert functional group, preventing precursor adsorption through chemical passivation and steric shielding [45]. SMIs' higher volatility allows vapor-phase delivery, improving selectivity in sub-10 nm, high-aspect-ratio structures. For effective AS-ALD using SMIs, it is crucial to design SMI-precursor pairs that exhibit strong reactivity with the substrate without reacting with the precursor. Although SAMs offer strong selectivity, their inherent limitations restrict their broader applicability, while SMIs are emerging as a promising alternative for future AS-ALD research.
4. Conclusion
-
Funding
This work has supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (NRF-2023R1A2C1006831).
-
Conflict of Interest
Byung Joon Choi serves as an editor of the Science editing, but has no role in the decision to publish this article. Except for that, no potential conflict of interest relevant to this article was reported.
-
Data Availability Statement
Data will be made available on request.
-
Author Information and Contribution
Si Eun Jung: Student; conceptualization, investigation, visualization, writing–original draft
Ji Woong Shin: Student; visualization, writing-original draft
Ye Jin Han: Student; visualization, writing-original draft
Byung Joon Choi: Professor; supervision, writing–review & editing
-
Acknowledgments
None.
Article information







| Head Group | SAM | Precursor | Temperature (℃) | NGS | GS | Reference |
|---|---|---|---|---|---|---|
| Thiol | ODT, DDT (solution) | DEZ, TIP | 120 | CuO | SiO2 | [1] |
| UDT, ODT (solution) | TDMAHf | 170 | CuO | SiO2 | [2] | |
| ODT | TDMAHf | 130~200 | Cu, CuO, Cu2O | SiO2 | [3] | |
| 1-dodecanethiol (DDT),1-octanethiol, and ethanethiol | TMA, DEZ | 115 | Au | SiO2 | [4] | |
| DDT | DEZ | 120 | Cu | SiO2 | [5] | |
| Phosphonic acid | ODTS, ODPA, DDPA | TMA | 50~200 | Al2O3 | SiO2 | [6] |
| PA, ODPA, OPA, EPA, PPA (solution) | Ethylbenzyl (EBECHRu)c | 250 | SiO2/Si, TiN/ SiO2/Si, W/TiN/ SiO2/Si, line patterend TiN on SiO2 | SiO2 | [22] | |
| ODPA | TMA, DEZ | 120 | CuO, Co3O4, WO3, RuO2 | SiO2 | [7] | |
| ODPA | DEZ | 150 | Cu | SiO2 | [23] | |
| Alkyl silane | DETA, FAS, TMODS, PEDA (solution) | TiCl4 | 100, 140, 150 | TiN | SiO2 | [24] |
| ODTS | Pb(tmhd)2 | 160 | SiO2 | Si | [25] | |
| ODTS | Co(MeCp)2 | 300, 350 | SiO2 | SiO2 | [8] | |
| ODTS | Ti[OCH(CH3)2]4 | 150 | SiO2 | SiO2 | [17] | |
| ODTMS | TMA, DEZ | 200 | SiO2 | Cu | [9] | |
| Carboxyl | SA (solution) | DEZ | 100 | SAM-treated Cu, Co | Cu, Co | [26] |
| SA (solution) | DEZ | 70 | Cu | SiO2 | [27] |
| -OH | -NH2 | -CH3 | |
|---|---|---|---|
| H+ affinity | 13.90 | 14.89 | 17.85 |
| Barrier | 0.65 | 1.22 | 1.82 |
| Total energy | -1.44 | -0.94 | 0.09 |
- 1. F. S. Minaye Hashemi, B. R. Birchansky and S. F. Bent: ACS Appl. Mater. Interfaces, 8 (2016) 33264.Article
- 2. L. Lecordier, S. Herregods and S. Armini: J. Vac. Sci. Technol., A, 36 (2018) 031605.Article
- 3. M. Pasquali, S. De Gendt and S. Armini: Appli. Surf. Sci., 540 (2021) 148307.Article
- 4. M. D. Sampson, J. D. Emery, M. J. Pellin and A. B. Martinson: ACS Appl. Mater. Interfaces, 9 (2017) 33429.Article
- 5. F. S. M. Hashemi and S. F. Bent: Adv. Mater. Interfaces, 3 (2016) 1600464.Article
- 6. S.-G. Seo, B.-C. Yeo, S.-S Han, C.-M Yoon, J.-Y. Yang, J.-G. Yoon, C.-K. Yoo, H.-J. Kim, Y.-B. Lee, S.-J. Lee, J.-M. Myoung, H.-B.-R. Lee, W.-H. Kim, I.-K Oh and H.-J. Kim: ACS Appl. Mater. Interfaces, 9 (2017) 41607.Article
- 7. D. Bobb-Semple, K. L. Nardi, N. Draeger, D. M. Hausmann and S. F. Bent: Chem. Mater., 31 (2019) 1635.Article
- 8. F. Mastrangelo, G. Fioravanti, R. Quaresima, R. Vinci and E. Gherlone: J. Biomater. Nanobiotechnol., 2 (2011) 533.Article
- 9. C.-W. Kim, M.-S. Choi, J.-H. Sim, H.-J. Kim and C.-H. Choi: Appl. Surf. Sci., 663 (2024) 160033.Article
- 10. T. L. Liu, M. Harake and S. F. Bent: Adv. Mater. Interfaces, 10 (2023) 2202134.Article
- 11. A. J. M. Mackus, A. A. Bol and W. M. M. Kessels: Nanoscale, 6 (2014) 10941.Article
- 12. A. J. Mackus, M. J. Merkx and W. M. Kessels: Chem. Mater., 31 (2018) 2.ArticlePDF
- 13. Y.-J. Choi, H.-J. Kim, E.-C. Kim, H.-Y. Kang, J.-H. Park, Y.-R. Do, K.-W. Kwak and M.-H. Cho: ACS Appl. Mater. Interfaces, 15 (2023) 41170.ArticlePDF
- 14. Y. Xu and C. B. Musgrave: Chem. Mater., 16 (2004) 646.Article
- 15. A. Chandekar, S. K. Sengupta and J. E. Whitten: Appl. Surf. Sci., 256 (2010) 2742.Article
- 16. M. G. Cho, J. H. Go and B. J. Choi: J. Powder Mater., 30 (2023) 56.
- 17. J. Huang, M.-G. Lee, A. Lucero, L. Cheng and J.-Y. Kim: J. Phys. Chem. C, 118 (2014) 23306.Article
- 18. T. L. Liu and S. F. Bent: Chem. Mater., 33 (2021) 513.Article
- 19. J. Yarbrough, A. B. Shearer and S. F. Bent: J. Vac. Sci. Technol., A, 39 (2021) 021002.Article
- 20. W.-S. Chang, M.-J. Choi, J.-H. Kim, S.-H. Cho and K.-H. Whang, In: Proceedings of the Korean Society of Mechanical Engineers Spring Conference; (2003) p. 114.
- 21. M.-M. Sung: Electron. Mater. Lett., 3 (2007) 137.
- 22. S.-H. Lee, J.-M. Lee, J.-H. Lee, J. Kwak, S.-W. Chung and W.-H Kim: Mater. Lett., 328 (2022) 133187.Article
- 23. F. S. M. Hashemi, C. Prasittichai and S. F. Bent: J. Phys. Chem. C, 118 (2014) 10957.Article
- 24. L. Zheng, W. He, V. Spampinato, A. Franquet, S. Sergeant, S. D. Gendt and S. Armini: Langmuir, 36 (2020) 13144.Article
- 25. W.-Y. Lee, N. P. Dasgupta, O. Trejo, J.-R. Lee, J.-E. Hwang, T. Usui and F. B. Prinz: Langmuir, 26 (2010) 6845.Article
- 26. S. Satyarthy, M. Hasan Ul Iqbal, F. Abida, R. Nahar, A. J. Hauser, M. M. C. Cheng and A. Ghosh: Nanomaterials, 13 (2023) 2713.Article
- 27. L. E. López-González, J. Guerrero-Sánchez and H. Tiznado: Surf. Interfaces, 41 (2023) 103298.Article
- 28. F. S. Minaye Hashemi, C. Prasittichai and S. F. Bent: ACS Nano, 9 (2015) 8710.Article
- 29. W.-Y. Lee, C. C. Chao, X. Jiang, J.-E. Hwang, S. Bent and F. Prinz: ECS Trans., 16 (2008) 173.ArticlePDF
- 30. W.-Y. Lee and F. B. Prinz: J. Electrochem. Soc., 156 (2009) G125.Article
- 31. C. Vericat, M. E. Vela, G. Benitez, P. Carro and R. C. Salvarezza: Chem. Soc. Rev., 39 (2010) 1805.Article
- 32. Y.-S. Chi, S.-M. Kang and I.-S. Choi: Polymer Sci. Technol., 17 (2006) 172.
- 33. R. Chen, H.-S. Kim, P. C. McIntyre and S. F. Bent: Chem. Mater., 17 (2005) 536.Article
- 34. J. Yu, Y. Lou, Z. Wang and G. Huang: Surf. Coat. Technol., 485 (2024) 130933.Article
- 35. S. M. Kanan, W. T. Tze and C. P. Tripp: Langmuir, 18 (2002) 6623.Article
- 36. I.-K. Oh, T. E. Sandoval, T. L. Liu, N. E. Richey and S. F. Bent: Chem. Mater., 33 (2021) 3926.Article
- 37. J. Soethoudt, Y. Tomczak, B. Meynaerts, B. T. Chan and A. Delabie: J. Phys. Chem. C, 124 (2020) 7163.Article
- 38. H.-G. Kim, M.-S. Kim, B.-W. Gu, M.-R. Khan, B.-G. Ko, S. Yasmeen, C.-S Kim, S.-H. Kwon, J.-Y. Kim, J.-H. Kwon, K.-S. Jin, B.-C. Cho, J.-S. Chun, B. Shong and H.-B.-R. Lee: Chem. Mater., 32 (2020) 8921.Article
- 39. Z. Guo, W. Zheng, H. Hamoudi, C. Dablemont, V. A. Esaulov and B. Bourguignon: Surf. Sci., 602 (2008) 3551.Article
- 40. H.-H. Jo, S.-H. Lee, E.-S. Youn, J.-E. Seo, J.-W. Lee, D.-H. Han, S.-A. Nam and J.-H. Han: J. Powder Mater., 30 (2023) 53.Article
- 41. A. Mameli and A. V. Teplyakov: Acc. Chem. Res., 56 (2023) 2084.ArticlePDF
- 42. R. Chen and S. F. Bent: Chem. Mater., 18 (2006) 3733.Article
- 43. N. Herzer, S. Hoeppener and U. S. Schubert: Chem. Commun., 46 (2010) 5634.Article
- 44. X. Wan, I. Lieberman, A. Asyuda, S. Resch, H. Seim, P. Kirsch and M. Zharnikov: J. Phys. Chem. C, 124 (2020) 2531.Article
- 45. M. J. Merkx, A. Angelidis, A. Mameli, J. Li, P. C. Lemaire, K. Sharma, D. M. Hausmann, W. M. M. Kessels, T. E. Sandoval and A. J. M. Mackus: J. Phys. Chem. C, 126 (2022) 4845.ArticlePDF
References
Figure & Data
References
Citations

- Temperature-Dependent Surface Structural Change in Self-Assembled Monolayers Studied with Vibrational Sum-Frequency Generation and QM/MD Simulation
Hojeong Yoon, Saima Sadiq, Junhyeok Park, Kyungwon Kwak, Minhaeng Cho
The Journal of Physical Chemistry Letters.2025;[Epub] CrossRef
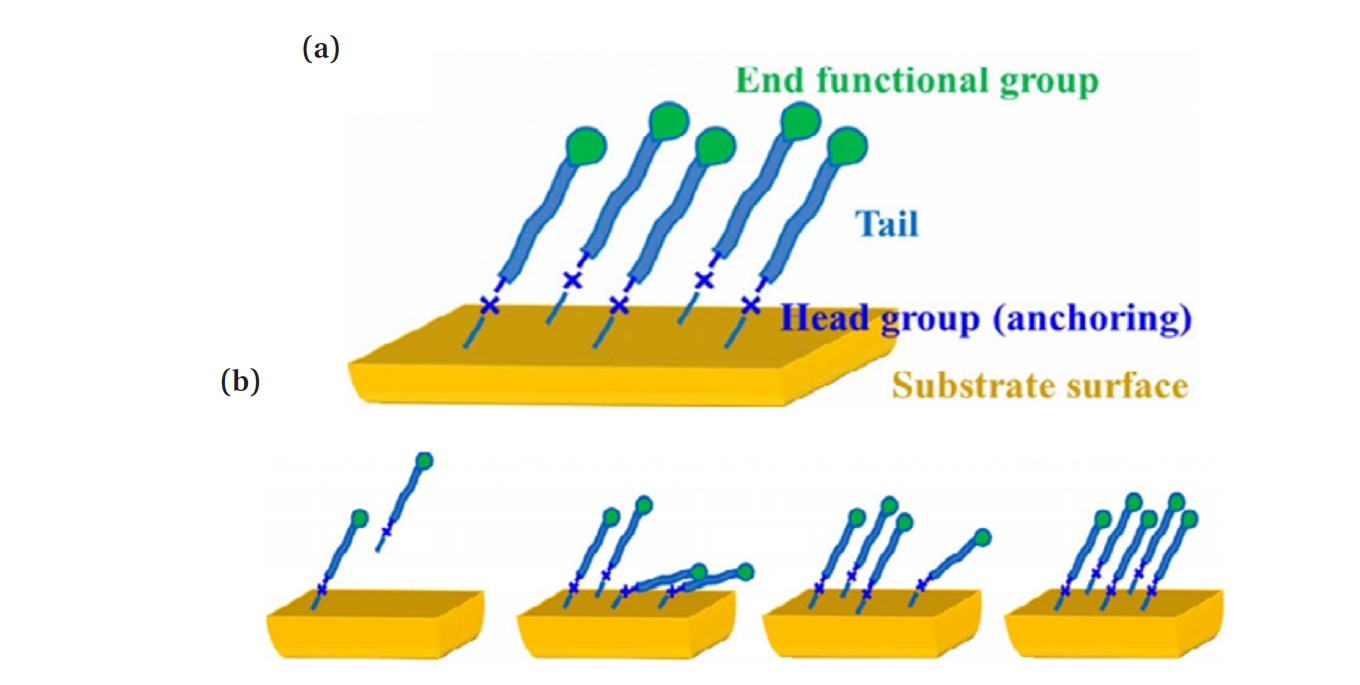
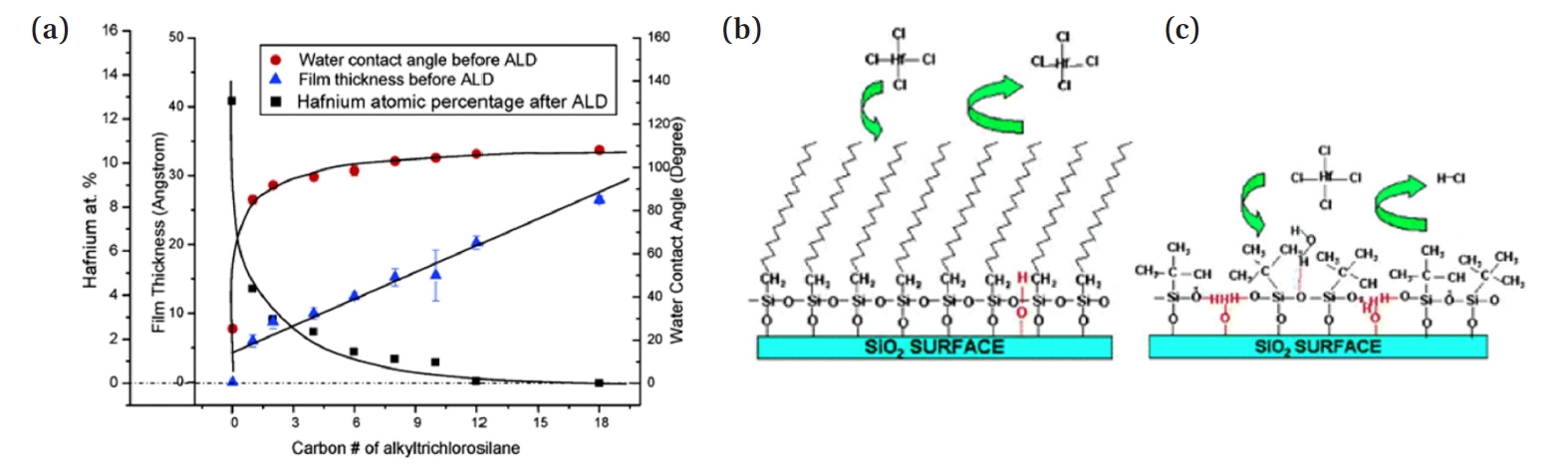


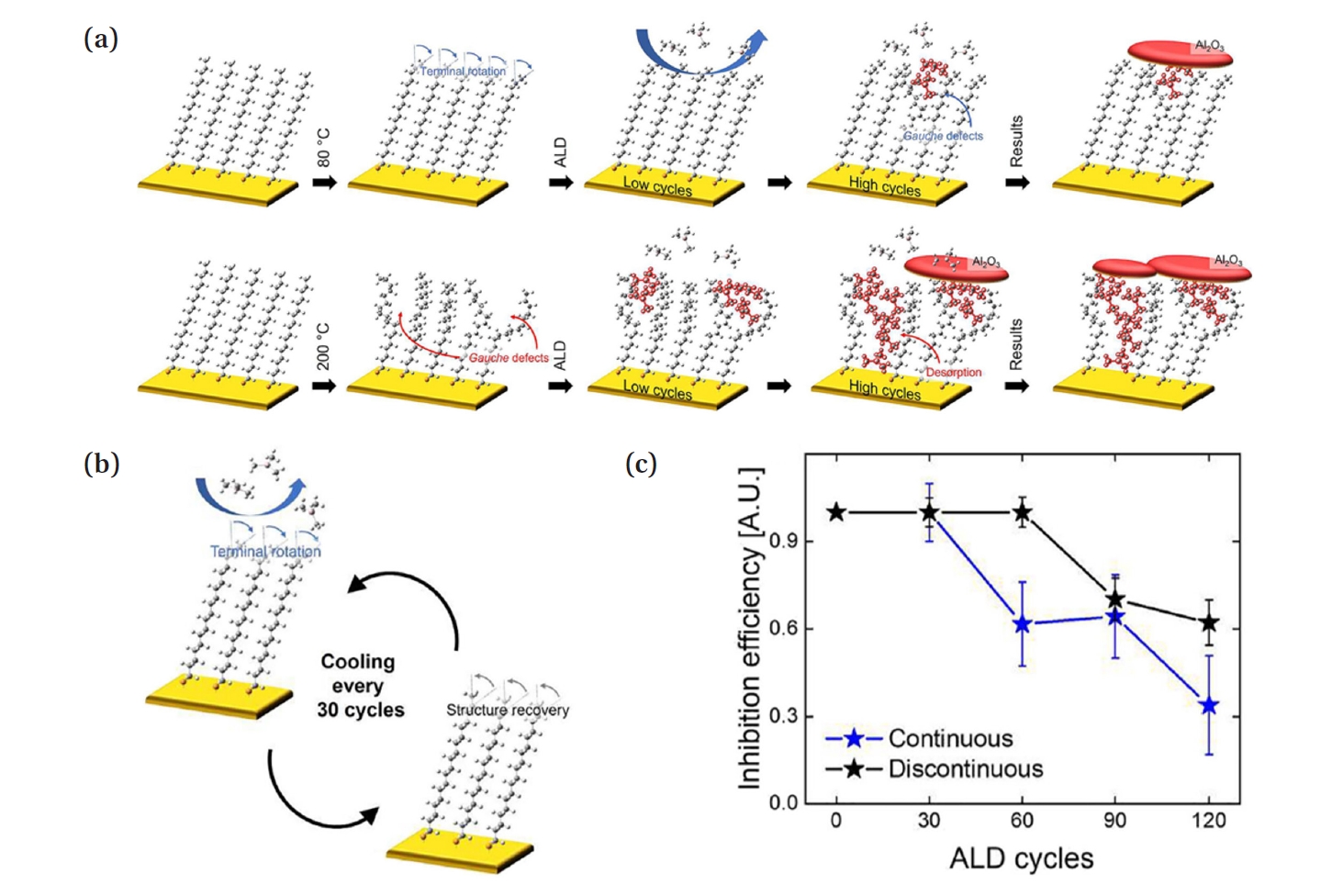
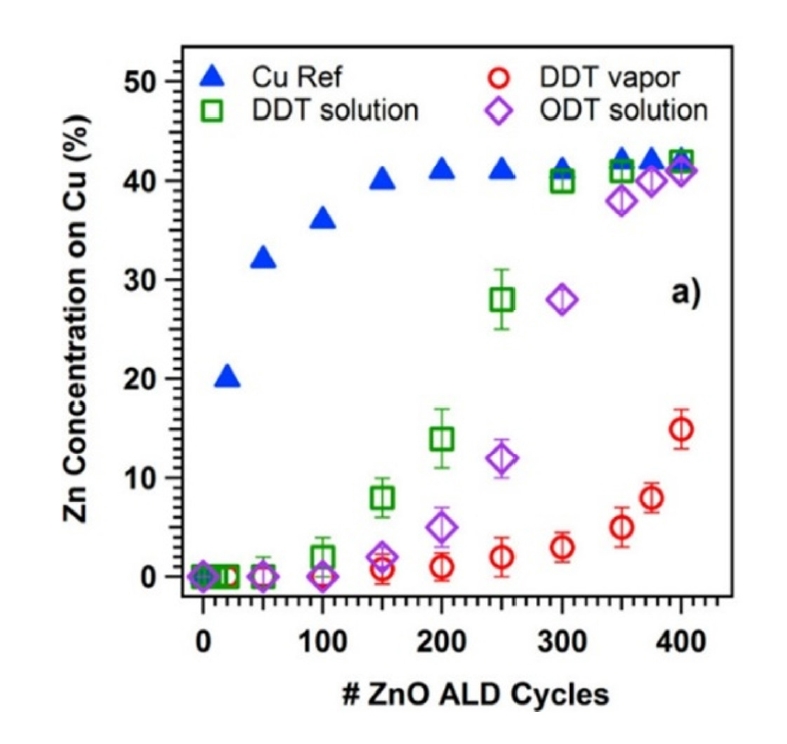
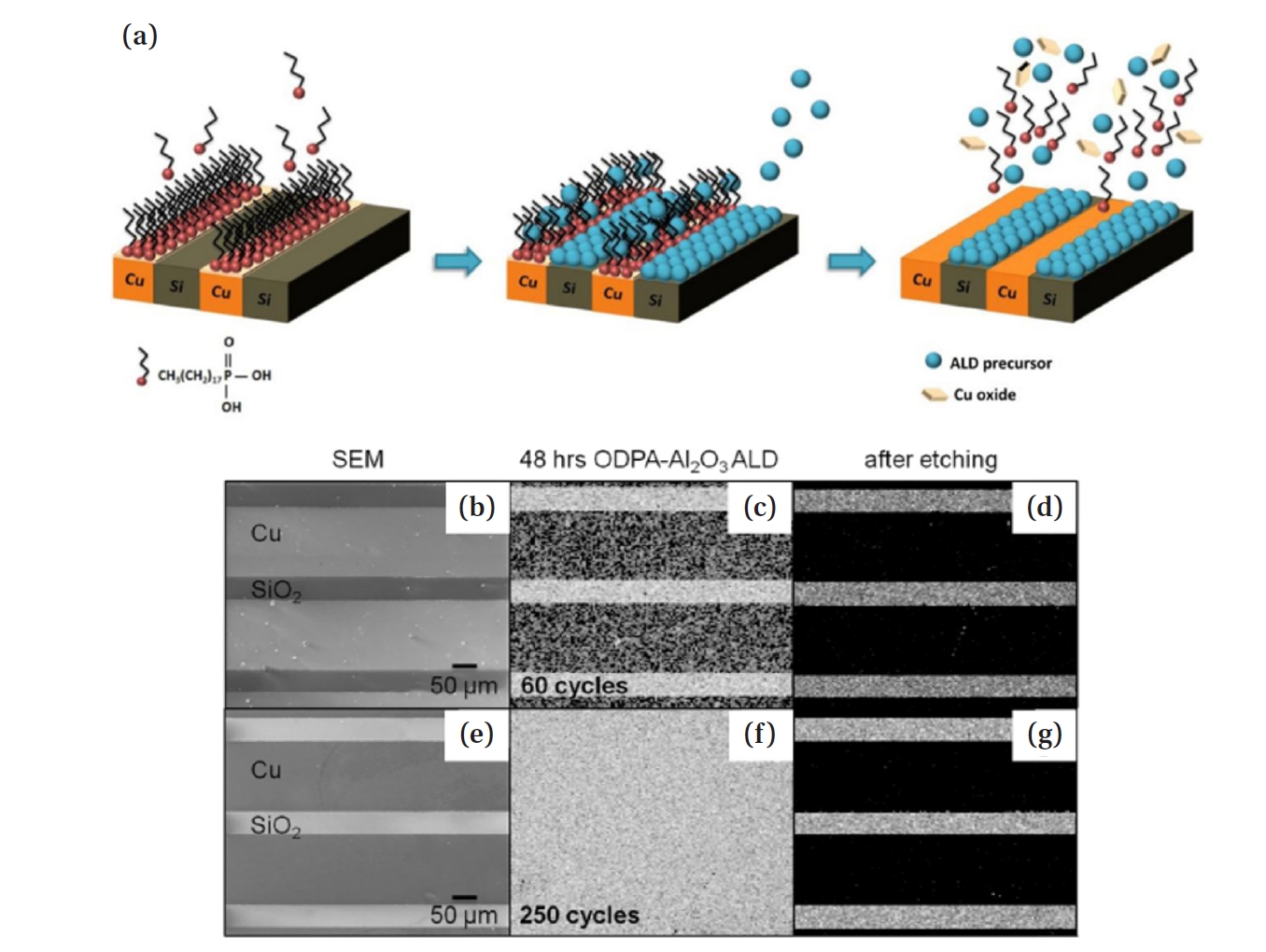
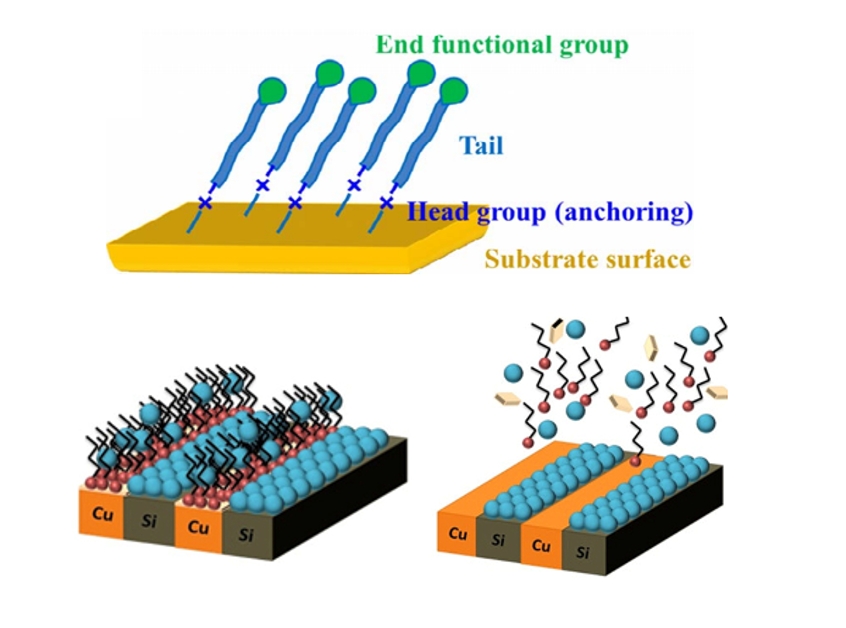
Fig. 1.
Fig. 2.
Fig. 3.
Fig. 4.
Fig. 5.
Fig. 6.
Fig. 7.
Graphical abstract
| Head Group | SAM | Precursor | Temperature (℃) | NGS | GS | Reference |
|---|---|---|---|---|---|---|
| Thiol | ODT, DDT (solution) | DEZ, TIP | 120 | CuO | SiO2 | [1] |
| UDT, ODT (solution) | TDMAHf | 170 | CuO | SiO2 | [2] | |
| ODT | TDMAHf | 130~200 | Cu, CuO, Cu2O | SiO2 | [3] | |
| 1-dodecanethiol (DDT),1-octanethiol, and ethanethiol | TMA, DEZ | 115 | Au | SiO2 | [4] | |
| DDT | DEZ | 120 | Cu | SiO2 | [5] | |
| Phosphonic acid | ODTS, ODPA, DDPA | TMA | 50~200 | Al2O3 | SiO2 | [6] |
| PA, ODPA, OPA, EPA, PPA (solution) | Ethylbenzyl (EBECHRu)c | 250 | SiO2/Si, TiN/ SiO2/Si, W/TiN/ SiO2/Si, line patterend TiN on SiO2 | SiO2 | [22] | |
| ODPA | TMA, DEZ | 120 | CuO, Co3O4, WO3, RuO2 | SiO2 | [7] | |
| ODPA | DEZ | 150 | Cu | SiO2 | [23] | |
| Alkyl silane | DETA, FAS, TMODS, PEDA (solution) | TiCl4 | 100, 140, 150 | TiN | SiO2 | [24] |
| ODTS | Pb(tmhd)2 | 160 | SiO2 | Si | [25] | |
| ODTS | Co(MeCp)2 | 300, 350 | SiO2 | SiO2 | [8] | |
| ODTS | Ti[OCH(CH3)2]4 | 150 | SiO2 | SiO2 | [17] | |
| ODTMS | TMA, DEZ | 200 | SiO2 | Cu | [9] | |
| Carboxyl | SA (solution) | DEZ | 100 | SAM-treated Cu, Co | Cu, Co | [26] |
| SA (solution) | DEZ | 70 | Cu | SiO2 | [27] |
| -OH | -NH2 | -CH3 | |
|---|---|---|---|
| H+ affinity | 13.90 | 14.89 | 17.85 |
| Barrier | 0.65 | 1.22 | 1.82 |
| Total energy | -1.44 | -0.94 | 0.09 |
| AlCl3 | AlCH3Cl2 | Al(CH3)2Cl | Al(CH3)3 | Al(C2H5)3 | |
|---|---|---|---|---|---|
| ΔH (kJ/mol) | 113.4 | 111.8 | 111.7 | 66.6 | 73.4 |
| -TΔS (kJ/mol) | -87.9 | -93.6 | -88.9 | -97.5 | -103.1 |
| ΔG (kJ/mol) | 25.6 | 18.3 | 22.9 | -31.0 | -29.7 |
| Dissociated dimer fraction (x) | 0.010 | 0.035 | 0.012 | 0.998 | 0.998 |
| Effective average size (Veff, Å3) | 143.7 | 147.6 | 151.6 | 87.2 | 140.2 |
Table 1.
Table 2.
Table 3.
TOP
 KPMI
KPMI


 ePub Link
ePub Link Cite this Article
Cite this Article